Interleukin-7 up-regulates cyclin D1 via activator protein-1 to promote proliferation of cell in lung cancer
- PMID: 21847632
- PMCID: PMC3249162
- DOI: 10.1007/s00262-011-1078-3
Interleukin-7 up-regulates cyclin D1 via activator protein-1 to promote proliferation of cell in lung cancer
Abstract
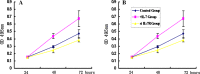
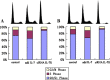
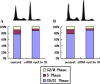
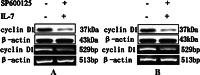
Interleukin-7 is a potent regulator of lymphocyte proliferation, but it inducing growth of solid tumors is few known. We study the relationship between Interleukin-7 and the regulator of the cell cycle, cyclin D1 and the mechanism of Interleukin-7 regulating cell growth in human lung cancer. We detected expression of cyclin D1 and its impact on the prognosis of lung cancer patients. Using Western blot, reverse transcriptase-PCR, Co-Immunoprecipitation, and Chromatin Immunoprecipitation, we investigated how Interleukin-7 regulated cyclin D1 in vitro and in nude mice. We found that, in lung cancer cell lines and in nude mice, Interleukin-7/Interleukin-7 receptor increased the expression of cyclin D1 and phosphorylation of c-Fos/c-Jun, induce c-Fos and c-Jun heterodimer formation, and enhanced c-Fos/c-Jun DNA-binding activity to regulate cyclin D1. In addition, lymph node metastasis, tumor stage, and cyclin D1 were the strongest predictors of survival in 100 human non-small cell lung cancer specimens analyzed. Taken together, our results provided evidence that Interleukin-7/Interleukin-7 receptor induced cyclin D1 up-regulation via c-Fos/c-Jun pathway to promote proliferation of cells in lung cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
[Interleukin 7 and its receptor promote cell proliferation and induce lymphangiogenesis in non-small cell lung cancer].Zhonghua Bing Li Xue Za Zhi. 2012 Aug;41(8):511-8. doi: 10.3760/cma.j.issn.0529-5807.2012.08.002. Zhonghua Bing Li Xue Za Zhi. 2012. PMID: 23157741 Chinese.
-
Interleukin 7/interleukin 7 receptor induce c-Fos/c-Jun-dependent vascular endothelial growth factor-D up-regulation: a mechanism of lymphangiogenesis in lung cancer.Eur J Cancer. 2009 Mar;45(5):866-73. doi: 10.1016/j.ejca.2008.12.006. Epub 2009 Jan 10. Eur J Cancer. 2009. PMID: 19136250
-
PAX2 protein induces expression of cyclin D1 through activating AP-1 protein and promotes proliferation of colon cancer cells.J Biol Chem. 2012 Dec 28;287(53):44164-72. doi: 10.1074/jbc.M112.401521. Epub 2012 Nov 7. J Biol Chem. 2012. PMID: 23135283 Free PMC article.
-
Circular RNA circ-CMPK1 contributes to cell proliferation of non-small cell lung cancer by elevating cyclin D1 via sponging miR-302e.Mol Genet Genomic Med. 2020 Feb;8(2):e999. doi: 10.1002/mgg3.999. Epub 2019 Dec 21. Mol Genet Genomic Med. 2020. PMID: 31863641 Free PMC article.
-
Anti-lymphangiogenesis effects of a specific anti-interleukin 7 receptor antibody in lung cancer model in vivo.Mol Carcinog. 2015 Feb;54(2):148-55. doi: 10.1002/mc.22082. Epub 2013 Sep 24. Mol Carcinog. 2015. PMID: 24115038
Cited by
-
Interleukin-7 Contributes to the Invasiveness of Prostate Cancer Cells by Promoting Epithelial-Mesenchymal Transition.Sci Rep. 2019 May 6;9(1):6917. doi: 10.1038/s41598-019-43294-4. Sci Rep. 2019. PMID: 31061414 Free PMC article.
-
Interleukin 7 signaling prevents apoptosis by regulating bcl-2 and bax via the p53 pathway in human non-small cell lung cancer cells.Int J Clin Exp Pathol. 2014 Feb 15;7(3):870-81. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24695377 Free PMC article.
-
Roles of phosphatidylinositol 3-kinase regulatory subunit alpha, activator protein-1, and programmed cell death 4 in diagnosis of papillary thyroid carcinoma.Tumour Biol. 2016 May;37(5):6519-26. doi: 10.1007/s13277-015-4476-x. Epub 2015 Dec 4. Tumour Biol. 2016. PMID: 26637226
-
Novel endoplasmic reticulum stress-related gene signature unveils CDKN3 as a prognosticator in neuroblastoma.Transl Pediatr. 2025 Jul 31;14(7):1471-1488. doi: 10.21037/tp-2025-142. Epub 2025 Jul 28. Transl Pediatr. 2025. PMID: 40800177 Free PMC article.
-
Common gamma chain cytokines in combinatorial immune strategies against cancer.Immunol Lett. 2016 Jan;169:61-72. doi: 10.1016/j.imlet.2015.11.007. Epub 2015 Nov 17. Immunol Lett. 2016. PMID: 26597610 Free PMC article. Review.
References
-
- Appasamy PM. Biological and clinical implications of interleukin-7 and lymphopoiesis. Cytokines Cell Mol Ther. 1999;5:25–39. - PubMed
-
- González-García S, García-Peydró M, Martín-Gayo E, Ballestar E, Esteller M, Bornstein R, et al. CSL-MAML-dependent Notch1 signaling controls T lineage-specific IL-7R {alpha} gene expression in early human thymopoiesis and leukemia. J Exp Med. 2009;206:779–791. doi: 10.1084/jem.20081922. - DOI - PMC - PubMed
-
- Rajasuriar R, Booth D, Solomon A, Chua K, Spelman T, Gouillou M, et al. Biological determinants of immune reconstitution in HIV-infected patients receiving antiretroviral therapy: the role of interleukin 7 and interleukin 7 receptor α and microbial translocation. J Infect Dis. 2010;202:1254–1264. doi: 10.1086/656369. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

