Cancer theranostics with near-infrared light-activatable multimodal nanoparticles
- PMID: 21848277
- PMCID: PMC3196765
- DOI: 10.1021/ar200022e
Cancer theranostics with near-infrared light-activatable multimodal nanoparticles
Abstract
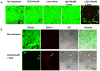
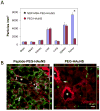
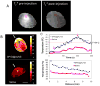
Nanomaterials that interact with light provide a unique opportunity for applications in biophotonic nanomedicine. Image-guided therapies could be designed based on multifunctional nanoparticles (NPs). Such NPs have a strong and tunable surface plasmon resonance absorption in the near-infrared region and can be detected using multiple imaging modalities (magnetic resonance imaging, nuclear imaging, and photoacoustic imaging). These novel nanostructures, once introduced, are expected to home in on solid tumors either via a passive targeting mechanism (i.e., the enhanced permeability and retention effect) or via an active targeting mechanism facilitated by ligands bound to their surfaces. Once the NPs reach their target tissue, their activity can then be turned on using an external stimulus. For example, photothermal conducting NPs primarily act by converting light energy into heat. As a result, the temperature in the treatment volume is elevated above the thermal damage threshold, which kills the cells. This process, termed photothermal ablation therapy (PTA), is effective, but it is also unlikely to kill all tumor cells when used alone. In addition to PTA, photothermal conducting NPs can also efficiently trigger the release of drugs and activate RNA interference. A multimodal approach, which permits simultaneous PTA therapy, chemotherapy, and therapeutic RNA interference, has the potential to completely eradicate residual diseased cells. In this Account, we provide an up-to-date review of the synthesis and characterization, functionalization, and in vitro and in vivo evaluation of NIR lightactivatable multifunctional nanostructures used for imaging and therapy. We emphasize research on hollow gold nanospheres, magnetic core-shell gold nanoshells, and semiconductor copper monosulfide NPs. We discuss three types of novel drug delivery systems in which hollow gold nanospheres are used to mediate controlled drug release.
Figures

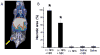

References
-
- Fiedler VU, Schwarzmaier HJ, Eickmeyer F, Muller FP, Schoepp C, Verreet PR. Laser-induced interstitial thermotherapy of liver metastases in an interventional 0. 5 Tesla MRI system: technique and first clinical experiences. J Magn Reson Imaging. 2001;13:729–737. - PubMed
-
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet. 1995;345:540–543. - PubMed
-
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787–800. - PubMed
-
- Rosenberg C, Puls R, Hegenscheid K, Kuehn J, Bollman T, Westerholt A, Weigel C, Hosten N. Laser ablation of metastatic lesions of the lung: long-term outcome. Am J Roentgenol. 2009;192:785–792. - PubMed
-
- Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SR, Atri M, Wilson BC, Fenster A, Trachtenberg J. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol. 2009;182:1371–1377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

