Isoprostane generation and function
- PMID: 21848345
- PMCID: PMC3192249
- DOI: 10.1021/cr200160h
Isoprostane generation and function
Figures
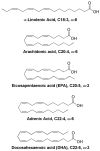
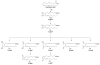
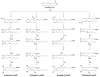
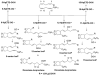
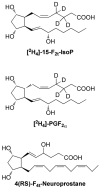
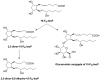

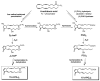
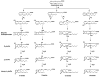
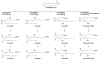

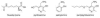
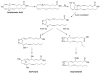


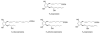
References
-
- Halliwell BG. Free Radicals in Biology and Medicine. 3rd. Oxford University Press; New York: 1999.
-
- Yin H, Porter NA. Antioxid Redox Signal. 2005;7:170. - PubMed
-
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, FitzGerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, R LJ, II, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Thiel DHV, Wellner D, Walter PB, Tome KB, Mason RP, Barrett JC. Free Radic Biol Med. 2005;38:698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

