Reactivity of N-(1,2,4-triazolyl)-substituted 1,2,3-triazoles
- PMID: 21848347
- PMCID: PMC3224851
- DOI: 10.1021/ol201949h
Reactivity of N-(1,2,4-triazolyl)-substituted 1,2,3-triazoles
Abstract
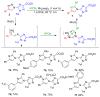
Synthetically useful rhodium(II) carbenes were obtained from N-(1,2,4-triazolyl)-substituted 1,2,3-triazoles and Rh(II) carboxylates. The electron-withdrawing 1,2,4-triazolyl group reveals the heretofore unknown reactivity of nonsulfonyl 1,2,3-triazoles, which exhibit the reactivity of diazo compounds. The resulting carbenes provide ready asymmetric access to secondary homoaminocyclopropanes (80-95% ee, dr >20:1) via reactions with olefins and also engage in efficient transannulation reactions with nitriles.
© 2011 American Chemical Society
Figures




References
-
-
α-Diazoimines are known to exist in cyclic 1,2,3-triazole form, except for those bearing strong electron-withdrawing group at N-1: Dimroth O. Ann. 1909;364:183.Gilchrist TL, Gymer GE. Adv Heterocycl Chem. 1974;16:33.
-
-
- Horneff T, Chuprakov S, Chernyak N, Gevorgyan V, Fokin VV. J Am Chem Soc. 2008;130:14972. - PMC - PubMed
- Grimster N, Zhang L, Fokin VV. J Am Chem Soc. 2010;132:2510. - PMC - PubMed
- Chuprakov S, Kwok SW, Zhang L, Lercher L, Fokin VV. J Am Chem Soc. 2009;131:18034. - PMC - PubMed
- Chuprakov S, Malik JA, Zibinsky M, Fokin VV. J Am Chem Soc. 2011;133:10352. - PMC - PubMed
-
- Doyle MP, McKervey M, Ye T. Modern catalytic methods for organic synthesis with diazo compounds: from cyclopropanes to ylides. Wiley; New York: 1998.
- Doyle MP. In: Reactive Intermediate Chemistry. Moss RA, Platz MS, Jones M Jr, editors. Wiley; New York: 2004. pp. 561–592.
- Davies HML, Manning JR. Nature. 2008;451:417. - PMC - PubMed
- Davies HML, Beckwith RE. Chem Rev. 2003;103:2861. - PubMed
- Doyle MP, Duffy R, Ratnikov M, Zhou L. Chem Rev. 2010;110:704. - PubMed
- Davies HML, Morton D. Chem Soc Rev. 2011;40:1857. - PubMed
-
- Xia F, Li W, Qu F, Fan Z, Liu X, Berro C, Rauzy E, Peng L. Org Biomol Chem. 2007;5:1695. - PubMed
-
- Espino CG, Fiori KW, Kim M, Du Bois J. J Am Chem Soc. 2004;126:15378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

