Inactivation of a Plasmodium apicoplast protein attenuates formation of liver merozoites
- PMID: 21848587
- PMCID: PMC3206223
- DOI: 10.1111/j.1365-2958.2011.07787.x
Inactivation of a Plasmodium apicoplast protein attenuates formation of liver merozoites
Abstract
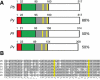
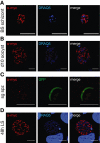
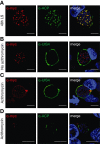
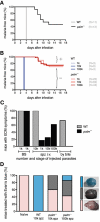
Malaria parasites undergo a population expansion inside the host liver before disease onset. Developmental arrest inside host hepatocytes elicits protective immune responses. Therefore, elucidation of the molecular mechanisms leading to mature hepatic merozoites, which initiate the pathogenic blood phase, also informs anti-malaria vaccine strategies. Using targeted gene deletion in the rodent model malaria parasite Plasmodium berghei, we show that a Plasmodium-specific Apicoplast protein plays an important role for Liver Merozoite formation (PALM). While the resulting knockout mutants develop normally for most of the life cycle, merozoite release into the blood stream and the ability to establish an infection are severely impaired. Presence of a signature blood-stage antigen, merozoite surface protein 1 and normal apicoplast morphology indicate that the inability to finalize merozoite segregation is a direct consequence of loss of PALM function. Experimental immunization of mice with as few as two doses of palm(-) sporozoites can elicit sterile protection up to 110 days after final immunization. Our data establish that a tailor-made arrest in the final steps of hepatic merozoite formation can induce strong protective immune responses and that malaria parasites employ a distinct apicoplast protein for efficient formation of pre-erythrocytic merozoites.
© 2011 Blackwell Publishing Ltd.
Figures


Similar articles
-
A Micronemal Protein, Scot1, Is Essential for Apicoplast Biogenesis and Liver Stage Development in Plasmodium berghei.ACS Infect Dis. 2024 Aug 9;10(8):3013-3025. doi: 10.1021/acsinfecdis.4c00362. Epub 2024 Jul 22. ACS Infect Dis. 2024. PMID: 39037752
-
Genetically modified Plasmodium parasites as a protective experimental malaria vaccine.Nature. 2005 Jan 13;433(7022):164-7. doi: 10.1038/nature03188. Epub 2004 Dec 5. Nature. 2005. PMID: 15580261
-
Overexpression of Plasmodium berghei ATG8 by Liver Forms Leads to Cumulative Defects in Organelle Dynamics and to Generation of Noninfectious Merozoites.mBio. 2016 Jun 28;7(3):e00682-16. doi: 10.1128/mBio.00682-16. mBio. 2016. PMID: 27353755 Free PMC article.
-
Parasite Recognition and Signaling Mechanisms in Innate Immune Responses to Malaria.Front Immunol. 2018 Dec 19;9:3006. doi: 10.3389/fimmu.2018.03006. eCollection 2018. Front Immunol. 2018. PMID: 30619355 Free PMC article. Review.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
Cited by
-
Murine infection models for vaccine development: the malaria example.Hum Vaccin Immunother. 2013 Mar;9(3):450-6. doi: 10.4161/hv.23218. Epub 2012 Dec 18. Hum Vaccin Immunother. 2013. PMID: 23249712 Free PMC article. Review.
-
Anti-malarial Drug Design by Targeting Apicoplasts: New Perspectives.J Pharmacopuncture. 2016 Mar;19(1):7-15. doi: 10.3831/KPI.2016.19.001. J Pharmacopuncture. 2016. PMID: 27280044 Free PMC article. Review.
-
Deletion of the rodent malaria ortholog for falcipain-1 highlights differences between hepatic and blood stage merozoites.PLoS Pathog. 2017 Sep 18;13(9):e1006586. doi: 10.1371/journal.ppat.1006586. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28922424 Free PMC article.
-
A Plasmodium Cross-Stage Antigen Contributes to the Development of Experimental Cerebral Malaria.Front Immunol. 2018 Aug 14;9:1875. doi: 10.3389/fimmu.2018.01875. eCollection 2018. Front Immunol. 2018. PMID: 30154793 Free PMC article.
-
Looking under the skin: the first steps in malarial infection and immunity.Nat Rev Microbiol. 2013 Oct;11(10):701-12. doi: 10.1038/nrmicro3111. Nat Rev Microbiol. 2013. PMID: 24037451 Review.
References
-
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Ménard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–224. - PubMed
-
- Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin. 2010;6:90–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

