A systematic review of treatment guidelines for metastatic colorectal cancer
- PMID: 21848897
- PMCID: PMC3562494
- DOI: 10.1111/j.1463-1318.2011.02765.x
A systematic review of treatment guidelines for metastatic colorectal cancer
Abstract
Aim: A systematic review of treatment guidelines for metastatic colorectal cancer (mCRC) was performed to assess recommendations for monoclonal antibody therapy in these guidelines.
Method: Relevant papers were identified through electronic searches of MEDLINE, MEDLINE In Process, EMBASE and the Cochrane Library; through manual searches of reference lists; and by searching the Internet.
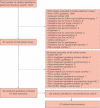
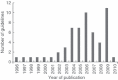
Results: A total of 57 relevant guidelines were identified, 32 through electronic database searches and 25 through the website searches. The majority of guidelines were published between 2004 and 2010. The country publishing the most guidelines was the USA (12), followed by the UK (10), Canada (eight), France (eight), Germany (three), Australia (two), Spain (two) and Italy (one). In addition, eight European and three international guidelines were identified. As monoclonal antibody therapy for mCRC was not introduced until 2004, no firm recommendations for monoclonal antibody therapy were made in guidelines published between 2004 and 2006. Recommendations for monoclonal antibody therapy first appeared in 2007 and evolved as more data became available. The most recent international, European and US guidelines recommend combination chemotherapy with the addition of a monoclonal antibody for the first-line treatment of mCRC. Second-line treatment depends on the first-line regimen used. For chemoresistant mCRC, cetuximab or panitumumab are recommended as monotherapy in patients with wild-type KRAS tumours.
Conclusion: The study indicates that recent treatment guidelines have recognized the role of monoclonal antibodies in the management of mCRC, and that treatment guidelines should be updated in a timely manner to reflect the most recently available data.
© 2011 The Authors. Colorectal Disease © 2011 The Association of Coloproctology of Great Britain and Ireland.
Figures
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. - PubMed
-
- Van Cutsem E, Oliveira J. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl. 4):iv61–3. - PubMed
-
- Yoo PS, Lopez-Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer. 2006;6:202–7. - PubMed
-
- Folprecht G, Grothey A, Alberts S, Raab HR, Kohne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–9. - PubMed
-
- De Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous