Localization of chondromodulin-I at the feto-maternal interface and its inhibitory actions on trophoblast invasion in vitro
- PMID: 21849085
- PMCID: PMC3171719
- DOI: 10.1186/1471-2121-12-34
Localization of chondromodulin-I at the feto-maternal interface and its inhibitory actions on trophoblast invasion in vitro
Abstract
Background: Chondromodulin-I (ChM-I) is an anti-angiogenic glycoprotein that is specifically localized at the extracellular matrix of the avascular mesenchyme including cartilage and cardiac valves. In this study, we characterized the expression pattern of ChM-I during early pregnancy in mice in vivo and its effect on invasion of trophoblastic cells into Matrigel in vitro.
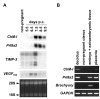
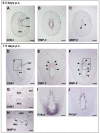
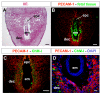
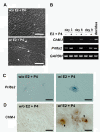
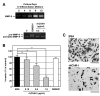
Results: Northern blot analysis clearly indicated that ChM-I transcripts were expressed in the pregnant mouse uterus at 6.5-9.5 days post coitum. In situ hybridization and immunohistochemistry revealed that ChM-I was localized to the mature decidua surrounding the matrix metalloproteinase-9 (MMP-9)-expressing trophoblasts. Consistent with this observation, the expression of ChM-I mRNA was induced in decidualizing endometrial stromal cells in vitro, in response to estradiol and progesterone. Recombinant human ChM-I (rhChM-I) markedly inhibited the invasion through Matrigel as well as the chemotactic migration of rat Rcho-1 trophoblast cells in a manner independent of MMP activation.
Conclusions: This study demonstrates the inhibitory action of ChM-I on trophoblast migration and invasion, implying the potential role of the ChM-I expression in decidual cells for the regulated tissue remodeling and angiogenesis at feto-maternal interface.
Figures



References
-
- Funaki H, Sawaguchi S, Yaoeda K, Koyama Y, Yaoita E, Funaki S, Shirakashi M, Oshima Y, Shukunami C, Hiraki Y. et al. Expression and localization of angiogenic inhibitory factor, chondromodulin-I, in adult rat eye. Invest Ophthalmol Vis Sci. 2001;42(6):1193–1200. - PubMed
-
- Hiraki Y, Inoue H, Iyama K, Kamizono A, Ochiai M, Shukunami C, Iijima S, Suzuki F, Kondo J. Identification of chondromodulin I as a novel endothelial cell growth inhibitor. Purification and its localization in the avascular zone of epiphyseal cartilage. J Biol Chem. 1997;272(51):32419–32426. doi: 10.1074/jbc.272.51.32419. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

