A phosphatase threshold sets the level of Cdk1 activity in early mitosis in budding yeast
- PMID: 21849476
- PMCID: PMC3183015
- DOI: 10.1091/mbc.E11-04-0340
A phosphatase threshold sets the level of Cdk1 activity in early mitosis in budding yeast
Abstract
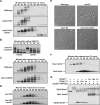
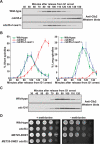
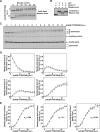
Entry into mitosis is initiated by synthesis of cyclins, which bind and activate cyclin-dependent kinase 1 (Cdk1). Cyclin synthesis is gradual, yet activation of Cdk1 occurs in a stepwise manner: a low level of Cdk1 activity is initially generated that triggers early mitotic events, which is followed by full activation of Cdk1. Little is known about how stepwise activation of Cdk1 is achieved. A key regulator of Cdk1 is the Wee1 kinase, which phosphorylates and inhibits Cdk1. Wee1 and Cdk1 show mutual regulation: Cdk1 phosphorylates Wee1, which activates Wee1 to inhibit Cdk1. Further phosphorylation events inactivate Wee1. We discovered that a specific form of protein phosphatase 2A (PP2A(Cdc55)) opposes the initial phosphorylation of Wee1 by Cdk1. In vivo analysis, in vitro reconstitution, and mathematical modeling suggest that PP2A(Cdc55) sets a threshold that limits activation of Wee1, thereby allowing a low constant level of Cdk1 activity to escape Wee1 inhibition in early mitosis. These results define a new role for PP2A(Cdc55) and reveal a systems-level mechanism by which dynamically opposed kinase and phosphatase activities can modulate signal strength.
Figures



References
-
- Amon A, Tyers M, Futcher B, Nasmyth K. Mechanisms that help the yeast cell cycle clock tick: G2 cyclins transcriptionally activate G2 cyclins and repress G1 cyclins. Cell. 1993;74:993–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

