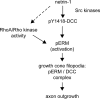
The activation of ezrin-radixin-moesin proteins is regulated by netrin-1 through Src kinase and RhoA/Rho kinase activities and mediates netrin-1-induced axon outgrowth
- PMID: 21849478
- PMCID: PMC3183026
- DOI: 10.1091/mbc.E10-11-0917
The activation of ezrin-radixin-moesin proteins is regulated by netrin-1 through Src kinase and RhoA/Rho kinase activities and mediates netrin-1-induced axon outgrowth
Abstract
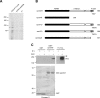
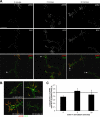
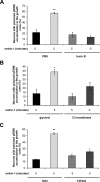
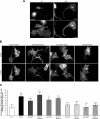
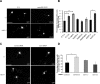
The receptor Deleted in Colorectal Cancer (DCC) mediates the attractive response of axons to the guidance cue netrin-1 during development. On netrin-1 stimulation, DCC is phosphorylated and induces the assembly of signaling complexes within the growth cone, leading to activation of cytoskeleton regulators, namely the GTPases Rac1 and Cdc42. The molecular mechanisms that link netrin-1/DCC to the actin machinery remain unclear. In this study we seek to demonstrate that the actin-binding proteins ezrin-radixin-moesin (ERM) are effectors of netrin-1/DCC signaling in embryonic cortical neurons. We show that ezrin associates with DCC in a netrin-1-dependent manner. We demonstrate that netrin-1/DCC induces ERM phosphorylation and activation and that the phosphorylation of DCC is required in that context. Moreover, Src kinases and RhoA/Rho kinase activities mediate netrin-1-induced ERM phosphorylation in neurons. We also observed that phosphorylated ERM proteins accumulate in growth cone filopodia, where they colocalize with DCC upon netrin-1 stimulation. Finally, we show that loss of ezrin expression in cortical neurons significantly decreases axon outgrowth induced by netrin-1. Together, our findings demonstrate that netrin-1 induces the formation of an activated ERM/DCC complex in growth cone filopodia, which is required for netrin-1-dependent cortical axon outgrowth.
Figures



References
-
- Charrin S, Alcover A. Role of ERM (ezrin-radixin-moesin) proteins in T lymphocyte polarization, immune synapse formation and in T cell receptor-mediated signaling. Front Biosci. 2006;11:1987–1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

