Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes
- PMID: 21849508
- PMCID: PMC3186378
- DOI: 10.1074/jbc.M111.285965
Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes
Abstract
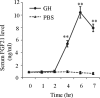
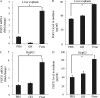
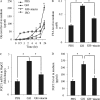
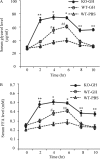
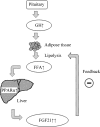
Fibroblast growth factor (FGF) 21 and growth hormone (GH) are metabolic hormones that play important roles in regulating glucose and lipid metabolism. Both hormones are induced in response to fasting and exert their actions on adipocytes to regulate lipolysis. However, the molecular interaction between these two hormones remains unclear. Here we demonstrate the existence of a feedback loop between GH and FGF21 on the regulation of lipolysis in adipocytes. A single bolus injection of GH into C57 mice acutely increases both mRNA and protein expression of FGF21 in the liver, thereby leading to a marked elevation of serum FGF21 concentrations. Such a stimulatory effect of GH on hepatic FGF21 production is abrogated by pretreatment of mice with the lipolysis inhibitor niacin. Direct incubation of either liver explants or human HepG2 hepatocytes with GH has no effect on FGF21 expression. On the other hand, FGF21 production in HepG2 cells is significantly induced by incubation with the conditioned medium harvested from GH-treated adipose tissue explants, which contains high concentrations of free fatty acids (FFA). Further analysis shows that FFA released by GH-induced lipolysis stimulates hepatic FGF21 expression by activation of the transcription factor PPARα. In FGF21-null mice, both the magnitude and duration of GH-induced lipolysis are significantly higher than those in their wild type littermates. Taken together, these findings suggest that GH-induced hepatic FGF21 production is mediated by FFA released from adipose tissues, and elevated FGF21 in turn acts as a negative feedback signal to terminate GH-stimulated lipolysis in adipocytes.
Figures



References
-
- Nishimura T., Nakatake Y., Konishi M., Itoh N. (2000) Biochim. Biophys. Acta 1492, 203–206 - PubMed
-
- Kharitonenkov A., Shanafelt A. B. (2009) Curr. Opin. Investig. Drugs. 10, 359–364 - PubMed
-
- Kharitonenkov A., Shiyanova T. L., Koester A., Ford A. M., Micanovic R., Galbreath E. J., Sandusky G. E., Hammond L. J., Moyers J. S., Owens R. A., Gromada J., Brozinick J. T., Hawkins E. D., Wroblewski V. J., Li D. S., Mehrbod F., Jaskunas S. R., Shanafelt A. B. (2005) J. Clin. Invest. 115, 1627–1635 - PMC - PubMed
-
- Badman M. K., Pissios P., Kennedy A. R., Koukos G,., Flier J. S., Maratos-Flier E. (2007) Cell Metab. 5, 426–437 - PubMed
-
- Coskun T., Bina H. A., Schneider M. A., Dunbar J. D., Hu C. C., Chen Y., Moller D. E., Kharitonenkov A. (2008) Endocrinology 149, 6018–6027 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

