Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage
- PMID: 21849535
- PMCID: PMC3164823
- DOI: 10.1523/JNEUROSCI.1887-11.2011
Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage
Abstract
Studies have linked sex-biased neurodevelopmental disorders, including autism and schizophrenia, with fetal antecedents such as prenatal stress. Further, these outcomes can persist into subsequent generations, raising the possibility that aspects of heritability in these diseases involve epigenetic mechanisms. Utilizing a mouse model in which we previously identified a period in early gestation when stress results in dysmasculinized and stress-sensitive male offspring, we have examined programming effects in second-generation offspring of prenatally stressed (F2-S) or control (F2-C) sires. Examination of gene expression patterns during the perinatal sensitive period, when organizational gonadal hormones establish the sexually dimorphic brain, confirmed dysmasculinization in F2-S males, where genes important in neurodevelopment showed a female-like pattern. Analyses of the epigenomic miRNA environment detected significant reductions in miR-322, miR-574, and miR-873 in the F2-S male brain, levels that were again more similar to those of control females. Increased expression of a common gene target for these three miRNAs, β-glycan, was confirmed in these males. These developmental effects were associated with the transmission of a stress-sensitive phenotype and shortened anogenital distance in adult F2-S males. As confirmation that the miRNA environment is responsive to organizational testosterone, neonatal males administered the aromatase inhibitor formestane exhibited dramatic changes in brain miRNA patterns, suggesting that miRNAs may serve a previously unappreciated role in organizing the sexually dimorphic brain. Overall, these data support the existence of a sensitive period of early gestation when epigenetic programming of the male germline can occur, permitting transmission of specific phenotypes into subsequent generations.
Figures
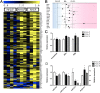

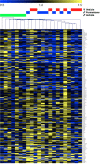
Comment in
-
Epigenetics: from father to son.Nat Rev Neurosci. 2011 Sep 7;12(10):548. doi: 10.1038/nrn3109. Nat Rev Neurosci. 2011. PMID: 21897431 No abstract available.
References
-
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. - PubMed
-
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. - PubMed
-
- Auger AP, Auger CJ. Epigenetic turn ons and turn offs: chromatin reorganization and brain differentiation. Endocrinology. 2011;152:349–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
