APP is phosphorylated by TrkA and regulates NGF/TrkA signaling
- PMID: 21849536
- PMCID: PMC3319322
- DOI: 10.1523/JNEUROSCI.1960-11.2011
APP is phosphorylated by TrkA and regulates NGF/TrkA signaling
Abstract
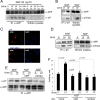
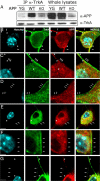
The pathogenic model of Alzheimer's disease (AD) posits that aggregates of amyloid β, a product of amyloid precursor protein (APP) processing, cause dementia. However, alterations of normal APP functions could contribute to AD pathogenesis, and it is therefore important to understand the role of APP. APP is a member of a gene family that shows functional redundancy as documented by the evidence that single knock-out mice are viable, whereas mice with combined deletions of APP family genes die shortly after birth. A residue in the APP intracellular region, Y(682), is indispensable for these essential functions of APP. It is therefore important to identify pathways that regulate phosphorylation of Y(682) as well as the role of Y(682) in vivo. TrkA is associated with both phosphorylation of APP-Y(682) and alteration of APP processing, suggesting that tyrosine phosphorylation of APP links APP processing and neurotrophic signaling to intracellular pathways associated with cellular differentiation and survival. Here we have tested whether the NGF/TrkA signaling pathway is a physiological regulator of APP phosphorylation. We find that NGF induces tyrosine phosphorylation of APP, and that APP interacts with TrkA and this interaction requires Y(682). Unpredictably, we also uncover that APP, and specifically Y(682), regulates activation of the NGF/TrkA signaling pathway in vivo, the subcellular distribution of TrkA and the sensitivity of neurons to the trophic action of NGF. This evidence suggests that these two membrane protein's functions are strictly interconnected and that the NGF/TrkA signaling pathway is involved in AD pathogenesis and can be used as a therapeutic target.
Figures

References
-
- Bertrand E, Brouillet E, Caillé I, Bouillot C, Cole GM, Prochiantz A, Allinquant B. A short cytoplasmic domain of the amyloid precursor protein induces apoptosis in vitro and in vivo. Mol Cell Neurosci. 2001;18:503–511. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
