Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1
- PMID: 21849540
- PMCID: PMC6623197
- DOI: 10.1523/JNEUROSCI.0905-11.2011
Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1
Abstract
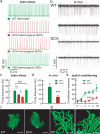
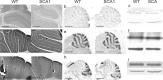
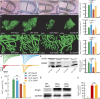
The contribution of neuronal dysfunction to neurodegeneration is studied in a mouse model of spinocerebellar ataxia type 1 (SCA1) displaying impaired motor performance ahead of loss or atrophy of cerebellar Purkinje cells. Presymptomatic SCA1 mice show a reduction in the firing rate of Purkinje cells (both in vivo and in slices) associated with a reduction in the efficiency of the main glutamatergic synapse onto Purkinje cells and with increased A-type potassium current. The A-type potassium channel Kv4.3 appears to be internalized in response to glutamatergic stimulation in Purkinje cells and accumulates in presymptomatic SCA1 mice. SCA1 mice are treated with aminopyridines, acting as potassium channel blockers to test whether the treatment could improve neuronal dysfunction, motor behavior, and neurodegeneration. In acutely treated young SCA1 mice, aminopyridines normalize the firing rate of Purkinje cells and the motor behavior of the animals. In chronically treated old SCA1 mice, 3,4-diaminopyridine improves the firing rate of Purkinje cells, the motor behavior of the animals, and partially protects against cell atrophy. Chronic treatment with 3,4-diaminopyridine is associated with increased cerebellar levels of BDNF, suggesting that partial protection against atrophy of Purkinje cells is possibly provided by an increased production of growth factors secondary to the reincrease in electrical activity. Our data suggest that aminopyridines might have symptomatic and/or neuroprotective beneficial effects in SCA1, that reduction in the firing rate of Purkinje cells can cause cerebellar ataxia, and that treatment of early neuronal dysfunction is relevant in neurodegenerative disorders such as SCA1.
Figures






References
-
- Adcock KH, Metzger F, Kapfhammer JP. Purkinje cell dendritic tree development in the absence of excitatory neurotransmission and of brain-derived neurotrophic factor in organotypic slice cultures. Neuroscience. 2004;127:137–145. - PubMed
-
- Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS, Duvick LA, Zoghbi HY, Orr HT. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–948. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
