The postnatal accumulation of junctional E-cadherin is inversely correlated with the capacity for supporting cells to convert directly into sensory hair cells in mammalian balance organs
- PMID: 21849546
- PMCID: PMC3164812
- DOI: 10.1523/JNEUROSCI.2525-11.2011
The postnatal accumulation of junctional E-cadherin is inversely correlated with the capacity for supporting cells to convert directly into sensory hair cells in mammalian balance organs
Abstract
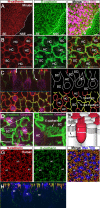
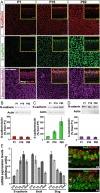
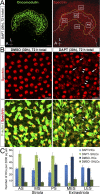
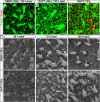
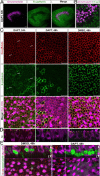
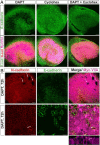
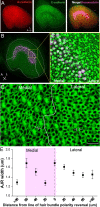
Mammals experience permanent impairments from hair cell (HC) losses, but birds and other non-mammals quickly recover hearing and balance senses after supporting cells (SCs) give rise to replacement HCs. Avian HC epithelia express little or no E-cadherin, and differences in the thickness of F-actin belts at SC junctions strongly correlate with different species' capacities for HC replacement, so we investigated junctional cadherins in human and murine ears. We found strong E-cadherin expression at SC-SC junctions that increases more than sixfold postnatally in mice. When we cultured utricles from young mice with γ-secretase inhibitors (GSIs), striolar SCs completely internalized their E-cadherin, without affecting N-cadherin. Hes and Hey expression also decreased and the SCs began to express Atoh1. After 48 h, those SCs expressed myosins VI and VIIA, and by 72 h, they developed hair bundles. However, some scattered striolar SCs retained E-cadherin and the SC phenotype. In extrastriolar regions, the vast majority of SCs also retained E-cadherin and failed to convert into HCs even after long GSI treatments. Microscopic measurements revealed that the junctions between extrastriolar SCs were more developed than those between striolar SCs. In GSI-treated utricles as old as P12, differentiated striolar SCs converted into HCs, but such responses declined with age and ceased by P16. Thus, temporal and spatial differences in postnatal SC-to-HC phenotype conversion capacity are linked to the structural attributes of E-cadherin containing SC junctions in mammals, which differ substantially from their counterparts in non-mammalian vertebrates that readily recover from hearing and balance deficits through hair cell regeneration.
Figures


References
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. - PubMed
-
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. - PubMed
-
- Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–756. - PubMed
-
- Chausovsky A, Bershadsky AD, Borisy GG. Cadherin-mediated regulation of microtubule dynamics. Nat Cell Biol. 2000;2:797–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
