Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction
- PMID: 21849683
- PMCID: PMC3169715
- DOI: 10.4049/jimmunol.1101649
Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction
Abstract
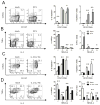
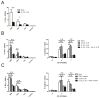
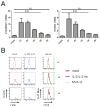
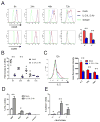
Regulatory T cells (Treg) are key players in maintaining immune homeostasis but have also been shown to regulate immune responses against infectious pathogens. Therefore, Treg are a promising target for modulating immune responses to vaccines to improve their efficacy. Using a viral vector system, we found that Treg act on the developing immune response early postinfection by reducing the extent of dendritic cell costimulatory molecule expression. Due to this change and the lower IL-2 production that results, a substantial fraction of CD8(+) effector T cells lose CD25 expression several days after activation. Surprisingly, such Treg-dependent limitations in IL-2 signaling by Ag-activated CD8(+) T cells prevent effector differentiation without interfering with memory cell formation. In this way, Treg fine-tune the numbers of effector T cells generated while preserving the capacity for a rapid recall response upon pathogen re-exposure. This selective effect of Treg on a subpopulation of CD8(+) T cells indicates that although manipulation of the Treg compartment might not be optimal for prophylactic vaccinations, it can be potentially exploited to optimize vaccine efficacy for therapeutic interventions.
Figures


References
-
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. - PubMed
-
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. - PubMed
-
- Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. - PubMed
-
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

