O-GlcNAc signalling: implications for cancer cell biology
- PMID: 21850036
- PMCID: PMC3291174
- DOI: 10.1038/nrc3114
O-GlcNAc signalling: implications for cancer cell biology
Abstract
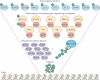
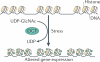
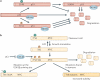
O-GlcNAcylation is the covalent attachment of β-D-N-acetylglucosamine (GlcNAc) sugars to serine or threonine residues of nuclear and cytoplasmic proteins, and it is involved in extensive crosstalk with other post-translational modifications, such as phosphorylation. O-GlcNAcylation is becoming increasing realized as having important roles in cancer-relevant processes, such as cell signalling, transcription, cell division, metabolism and cytoskeletal regulation. However, currently little is known about the specific roles of aberrant O-GlcNAcylation in cancer. In this Opinion article, we summarize the current understanding of O-GlcNAcylation in cancer and its emerging functions in transcriptional regulation at the level of chromatin and transcription factors.
Figures
References
-
- Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984;259:3308–3317. - PubMed
-
- Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

