Molecular characterization of transcriptome-wide interactions between highly pathogenic porcine reproductive and respiratory syndrome virus and porcine alveolar macrophages in vivo
- PMID: 21850204
- PMCID: PMC3157269
- DOI: 10.7150/ijbs.7.947
Molecular characterization of transcriptome-wide interactions between highly pathogenic porcine reproductive and respiratory syndrome virus and porcine alveolar macrophages in vivo
Erratum in
- Int J Biol Sci. 2012;8(1):124
Abstract
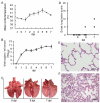
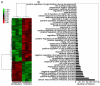
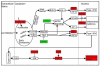
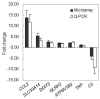
Porcine reproductive and respiratory syndrome virus (PRRSV) infects mainly the porcine alveolar macrophages (PAMs) and causes porcine reproductive and respiratory syndrome (PRRS). Previous studies have analyzed the global gene expression profiles of lung tissue in vivo and PAMs in vitro following infection with PRRSV, however, transcriptome-wide understanding of the interaction between highly pathogenic PRRSV (HP-PRRSV) and PAMs in vivo has not yet been established. In this study, we employed Affymetrix microarrays to investigate the gene expression patterns of PAMs isolated from Tongcheng piglets (a Chinese indigenous breed) after infection with HP-PRRSV. During the infection, Tongcheng piglets exhibited typical clinical signs, e.g. fever, asthma, coughing, anorexia, lethargy and convulsion, but displayed mild regional lung damage at 5 and 7 dpi. Microarray analysis revealed that HP-PRRSV infection has affected PAMs in expression of the important genes involved in cytoskeleton and exocytosis organization, protein degradation and folding, intracellular calcium and zinc homeostasis. Several potential antiviral strategies might be employed in PAMs, including upregulating IFN-induced genes and increasing intracellular zinc ion concentration. And inhibition of the complement system likely attenuated the lung damage during HP-PRRSV infection. Transcriptomic analysis of PAMs in vivo could lead to a better understanding of the HP-PRRSV-host interaction, and to the identification of novel antiviral therapies and genetic components of swine tolerance/susceptibility to HP-PRRS.
Keywords: HP-PRRSV-host interaction; antiviral strategy; infection; microarray; pulmonary alveolar macrophage.
Conflict of interest statement
Conflict of Interests: The authors have declared that no conflict of interest exists.
Figures
Similar articles
-
Transcriptome Analysis Reveals Dynamic Gene Expression Profiles in Porcine Alveolar Macrophages in Response to the Chinese Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus.Biomed Res Int. 2018 Apr 29;2018:1538127. doi: 10.1155/2018/1538127. eCollection 2018. Biomed Res Int. 2018. PMID: 29854728 Free PMC article.
-
Transcriptome Differences in Porcine Alveolar Macrophages from Tongcheng and Large White Pigs in Response to Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Infection.Int J Mol Sci. 2017 Jul 12;18(7):1475. doi: 10.3390/ijms18071475. Int J Mol Sci. 2017. PMID: 28704922 Free PMC article.
-
The gene expression profile of porcine alveolar macrophages infected with a highly pathogenic porcine reproductive and respiratory syndrome virus indicates overstimulation of the innate immune system by the virus.Arch Virol. 2015 Mar;160(3):649-62. doi: 10.1007/s00705-014-2309-7. Epub 2014 Dec 12. Arch Virol. 2015. PMID: 25504361
-
Reappraising host cellular factors involved in attachment and entry to develop antiviral strategies against porcine reproductive and respiratory syndrome virus.Front Microbiol. 2022 Jul 26;13:975610. doi: 10.3389/fmicb.2022.975610. eCollection 2022. Front Microbiol. 2022. PMID: 35958155 Free PMC article. Review.
-
Research Progress on the detection methods of porcine reproductive and respiratory syndrome virus.Front Microbiol. 2023 Mar 9;14:1097905. doi: 10.3389/fmicb.2023.1097905. eCollection 2023. Front Microbiol. 2023. PMID: 36970703 Free PMC article. Review.
Cited by
-
RNA-sequence analysis of primary alveolar macrophages after in vitro infection with porcine reproductive and respiratory syndrome virus strains of differing virulence.PLoS One. 2014 Mar 18;9(3):e91918. doi: 10.1371/journal.pone.0091918. eCollection 2014. PLoS One. 2014. PMID: 24643046 Free PMC article.
-
LSM14A inhibits porcine reproductive and respiratory syndrome virus (PRRSV) replication by activating IFN-β signaling pathway in Marc-145.Mol Cell Biochem. 2015 Jan;399(1-2):247-56. doi: 10.1007/s11010-014-2251-8. Epub 2014 Nov 19. Mol Cell Biochem. 2015. PMID: 25408553
-
Genetic analysis of the blood transcriptome of young healthy pigs to improve disease resilience.Genet Sel Evol. 2023 Dec 12;55(1):90. doi: 10.1186/s12711-023-00860-9. Genet Sel Evol. 2023. PMID: 38087235 Free PMC article.
-
Interferon induced IFIT family genes in host antiviral defense.Int J Biol Sci. 2013;9(2):200-8. doi: 10.7150/ijbs.5613. Epub 2013 Feb 11. Int J Biol Sci. 2013. PMID: 23459883 Free PMC article. Review.
-
Comparative analysis of cytokine transcript profiles within mediastinal lymph node compartments of pigs after infection with porcine reproductive and respiratory syndrome genotype 1 strains differing in pathogenicity.Vet Res. 2015 Mar 19;46(1):34. doi: 10.1186/s13567-015-0161-8. Vet Res. 2015. PMID: 25889072 Free PMC article.
References
-
- Lewis CR, Torremorell M, Galina-Pantoja L, Bishop SC. Genetic parameters for performance traits in commercial sows estimated before and after an outbreak of porcine reproductive and respiratory syndrome. J Anim Sci. 2009;87:876–84. - PubMed
-
- Thanawongnuwech R, Halbur PG, Andrews JJ. Immunohistochemical detection of porcine reproductive and respiratory syndrome virus antigen in neurovascular lesions. J Vet Diagn Invest. 1997;9:334–7. - PubMed
-
- Murtaugh MP, Xiao Z, Zuckermann F. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol. 2002;15:533–47. - PubMed
-
- Cheon DS, Chae C. Distribution of a Korean strain of porcine reproductive and respiratory syndrome virus in experimentally infected pigs, as demonstrated immunohistochemically and by in-situ hybridization. J Comp Pathol. 1999;120:79–88. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

