High-throughput identification of potential minor histocompatibility antigens by MHC tetramer-based screening: feasibility and limitations
- PMID: 21850230
- PMCID: PMC3151248
- DOI: 10.1371/journal.pone.0022523
High-throughput identification of potential minor histocompatibility antigens by MHC tetramer-based screening: feasibility and limitations
Abstract
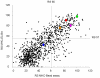
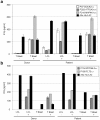
T-cell recognition of minor histocompatibility antigens (MiHA) plays an important role in the graft-versus-tumor (GVT) effect of allogeneic stem cell transplantation (allo-SCT). However, the number of MiHA identified to date remains limited, making clinical application of MiHA reactive T-cell infusion difficult. This study represents the first attempt of genome-wide prediction of MiHA, coupled to the isolation of T-cell populations that react with these antigens. In this unbiased high-throughput MiHA screen, both the possibilities and pitfalls of this approach were investigated. First, 973 polymorphic peptides expressed by hematopoietic stem cells were predicted and screened for HLA-A2 binding. Subsequently a set of 333 high affinity HLA-A2 ligands was identified and post transplantation samples from allo-SCT patients were screened for T-cell reactivity by a combination of pMHC-tetramer-based enrichment and multi-color flow cytometry. Using this approach, 71 peptide-reactive T-cell populations were generated. The isolation of a T-cell line specifically recognizing target cells expressing the MAP4K1(IMA) antigen demonstrates that identification of MiHA through this approach is in principle feasible. However, with the exception of the known MiHA HMHA1, none of the other T-cell populations that were generated demonstrated recognition of endogenously MiHA expressing target cells, even though recognition of peptide-loaded targets was often apparent. Collectively these results demonstrate the technical feasibility of high-throughput analysis of antigen-specific T-cell responses in small patient samples. However, the high-sensitivity of this approach requires the use of potential epitope sets that are not solely based on MHC binding, to prevent the frequent detection of T-cell responses that lack biological relevance.
Conflict of interest statement
Figures




References
-
- Porter DL, Roth MS, McGarigle C, Ferrara J, Antin JH. Induction of Graft-versus-Host Disease as Immunotherapy for Relapsed Chronic Myeloid Leukemia. N Engl J Med. 1994;330:100–106. - PubMed
-
- Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia [see comments]. Blood. 1995;86:2041–2050. - PubMed
-
- Marijt WAE, Heemskerk MHM, Kloosterboer FM, Goulmy E, Kester MGD, et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2742–2747. - PMC - PubMed
-
- Slager EH, Honders MW, van der Meijden ED, van Luxemburg-Heijs SAP, Kloosterboer FM, et al. Identification of the angiogenic endothelial-cell growth factor-1/thymidine phosphorylase as a potential target for immunotherapy of cancer. Blood. 2006;107:4954–4960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

