Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice
- PMID: 21850237
- PMCID: PMC3151263
- DOI: 10.1371/journal.pone.0022818
Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice
Abstract
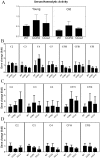
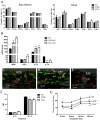
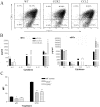
We have shown previously that a para-inflammatory response exists at the retinal/choroidal interface in the aging eye; and this response plays an important role in maintaining retinal homeostasis under chronic stress conditions. We hypothesized that dysregulation of the para-inflammatory response may result in an overt pro-inflammatory response inducing retinal degeneration. In this study, we examined this hypothesis in mice deficient in chemokine CCL2 or its cognate receptor CCR2. CCL2- or CCR2-deficient mice developed retinal degenerative changes with age, characterized as retinal pigment epithelial (RPE) cell and photoreceptor cell death. Retinal cell death was associated with significantly more subretinal microglial accumulation and increased complement activation. In addition, monocytes from CCL2- or CCR2-deficient mice had reduced capacity for phagocytosis and chemotaxis, expressed less IL-10 but more iNOS, IL-12 and TNF-α when compared to monocytes from WT mice. Complement activation at the site of RPE cell death resulted in C3b/C3d but not C5b-9 deposition, indicating only partial activation of the complement pathway. Our results suggest that altered monocyte functions may convert the protective para-inflammatory response into an overtly harmful inflammation at the retina/choroidal interface in CCL2- or CCR2-deficient mice, leading to RPE and photoreceptor degeneration. These data support a concept whereby a protective para-inflammatory response relies upon a normally functioning innate immune system. If the innate immune system is deficient chronic stress may tip the balance towards an overt inflammatory response causing cell/tissue damage.
Conflict of interest statement
Figures


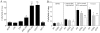
Similar articles
-
CCR2/CCL2-mediated inflammation protects photoreceptor cells from amyloid-β-induced apoptosis.Neurobiol Dis. 2011 Apr;42(1):55-72. doi: 10.1016/j.nbd.2011.01.004. Epub 2011 Jan 8. Neurobiol Dis. 2011. PMID: 21220018
-
Expression pattern of Ccr2 and Cx3cr1 in inherited retinal degeneration.J Neuroinflammation. 2015 Oct 12;12:188. doi: 10.1186/s12974-015-0408-3. J Neuroinflammation. 2015. PMID: 26458944 Free PMC article.
-
CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice.EMBO Mol Med. 2013 Nov;5(11):1775-93. doi: 10.1002/emmm.201302692. Epub 2013 Oct 21. EMBO Mol Med. 2013. PMID: 24142887 Free PMC article.
-
Para-inflammation in the aging retina.Prog Retin Eye Res. 2009 Sep;28(5):348-68. doi: 10.1016/j.preteyeres.2009.06.001. Epub 2009 Jun 26. Prog Retin Eye Res. 2009. PMID: 19560552 Review.
-
CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration.J Neuroinflammation. 2010 Dec 2;7:87. doi: 10.1186/1742-2094-7-87. J Neuroinflammation. 2010. PMID: 21126357 Free PMC article. Review.
Cited by
-
Age- and light-dependent development of localised retinal atrophy in CCL2(-/-)CX3CR1(GFP/GFP) mice.PLoS One. 2013 Apr 18;8(4):e61381. doi: 10.1371/journal.pone.0061381. Print 2013. PLoS One. 2013. PMID: 23637822 Free PMC article.
-
Deficiency of CC chemokine ligand 2 and decay-accelerating factor causes retinal degeneration in mice.Exp Eye Res. 2015 Sep;138:126-33. doi: 10.1016/j.exer.2015.05.016. Epub 2015 Jul 3. Exp Eye Res. 2015. PMID: 26149093 Free PMC article.
-
The Project MACULA Retinal Pigment Epithelium Grading System for Histology and Optical Coherence Tomography in Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2015 May;56(5):3253-68. doi: 10.1167/iovs.15-16431. Invest Ophthalmol Vis Sci. 2015. PMID: 25813989 Free PMC article.
-
Expression of complement components and regulators by different subtypes of bone marrow-derived macrophages.Inflammation. 2012 Aug;35(4):1448-61. doi: 10.1007/s10753-012-9458-1. Inflammation. 2012. PMID: 22450524
-
Attenuating Diabetic Vascular and Neuronal Defects by Targeting P2rx7.Int J Mol Sci. 2019 Apr 29;20(9):2101. doi: 10.3390/ijms20092101. Int J Mol Sci. 2019. PMID: 31035433 Free PMC article.
References
-
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. - PubMed
-
- Chakravarthy U, Adamis AP, Cunningham ET, Jr, Goldbaum M, et al. VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology. 2006;113(9):1508.e1–1508.25. - PubMed
-
- Hughes MS, Sang DN. Safety and efficacy of intravitreal bevacizumab followed by pegaptanib maintenance as a treatment regimen for age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2006;37(6):446–454. - PubMed
-
- Bressler NM, Chang TS, Fine JT, Dolan CM, Ward J, et al. Improved vision-related function after ranibizumab vs photodynamic therapy: a randomized clinical trial. Arch Ophthalmol. 2009;127(1):13–21. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

