Variability of bio-clinical parameters in Chinese-origin Rhesus macaques infected with simian immunodeficiency virus: a nonhuman primate AIDS model
- PMID: 21850259
- PMCID: PMC3151272
- DOI: 10.1371/journal.pone.0023177
Variability of bio-clinical parameters in Chinese-origin Rhesus macaques infected with simian immunodeficiency virus: a nonhuman primate AIDS model
Abstract
Background: Although Chinese-origin Rhesus macaques (Ch RhMs) infected with simian immunodeficiency virus (SIV) have been used for many years to evaluate the efficacy of AIDS vaccines and therapeutics, the bio-clinical variability of such a nonhuman primate AIDS model was so far not established.
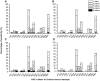
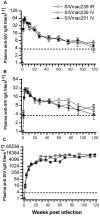
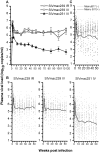
Methodology/principal findings: By randomizing 150 (78 male and 72 female) Ch RhMs with diverse MHC class I alleles into 3 groups (50 animals per group) challenged with intrarectal (i.r.) SIVmac239, intravenous (i.v.) SIVmac239, or i.v. SIVmac251, we evaluated variability in bio-clinical endpoints for 118 weeks. All SIV-challenged Ch RhMs became seropositive for SIV during 1-2 weeks. Plasma viral load (VL) peaked at weeks 1-2 and then declined to set-point levels as from week 5. The set-point VL was 30 fold higher in SIVmac239 (i.r. or i.v.)-infected than in SIVmac251 (i.v.)-infected animals. This difference in plasma VL increased overtime (>100 fold as from week 68). The rates of progression to AIDS or death were more rapid in SIVmac239 (i.r. or i.v.)-infected than in SIVmac251 (i.v.)-infected animals. No significant difference in bio-clinical endpoints was observed in animals challenged with i.r. or i.v. SIVmac239. The variability (standard deviation) in peak/set-point VL was nearly one-half lower in animals infected with SIVmac239 (i.r. or i.v.) than in those infected with SIVmac251 (i.v.), allowing that the same treatment-related difference can be detected with one-half fewer animals using SIVmac239 than using SIVmac251.
Conclusion/significance: These results provide solid estimates of variability in bio-clinical endpoints needed when designing studies using the Ch RhM SIV model and contribute to the improving quality and standardization of preclinical studies.
Conflict of interest statement
Figures

Similar articles
-
Viral factors determine progression to AIDS in simian immunodeficiency virus-infected newborn rhesus macaques.J Virol. 1995 Jul;69(7):4198-205. doi: 10.1128/JVI.69.7.4198-4205.1995. J Virol. 1995. PMID: 7769679 Free PMC article.
-
[Comparison of intravenous and intrarectal SIVmac239 infections in rhesus monkeys of Chinese origin].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2008 Apr;30(2):156-60. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2008. PMID: 18505116 Chinese.
-
Variability of viral load in plasma of rhesus monkeys inoculated with simian immunodeficiency virus or simian-human immunodeficiency virus: implications for using nonhuman primate AIDS models to test vaccines and therapeutics.J Virol. 2001 Nov;75(22):11234-8. doi: 10.1128/JVI.75.22.11234-11238.2001. J Virol. 2001. PMID: 11602764 Free PMC article.
-
Derivation and Characterization of Pathogenic Transmitted/Founder Molecular Clones from Simian Immunodeficiency Virus SIVsmE660 and SIVmac251 following Mucosal Infection.J Virol. 2016 Sep 12;90(19):8435-53. doi: 10.1128/JVI.00718-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27412591 Free PMC article.
-
SIV infection of rhesus macaques of Chinese origin: a suitable model for HIV infection in humans.Retrovirology. 2013 Aug 15;10:89. doi: 10.1186/1742-4690-10-89. Retrovirology. 2013. PMID: 23947613 Free PMC article. Review.
Cited by
-
Mucosal SIV Vaccines Comprising Inactivated Virus Particles and Bacterial Adjuvants Induce CD8(+) T-Regulatory Cells that Suppress SIV-Positive CD4(+) T-Cell Activation and Prevent SIV Infection in the Macaque Model.Front Immunol. 2014 Jun 30;5:297. doi: 10.3389/fimmu.2014.00297. eCollection 2014. Front Immunol. 2014. PMID: 25071760 Free PMC article.
-
Major histocompatibility complex class I haplotype diversity in Chinese rhesus macaques.G3 (Bethesda). 2013 Jul 8;3(7):1195-201. doi: 10.1534/g3.113.006254. G3 (Bethesda). 2013. PMID: 23696100 Free PMC article.
-
Control of SIV infection and subsequent induction of pandemic H1N1 immunity in rhesus macaques using an Ad5 [E1-, E2b-] vector platform.Vaccine. 2012 Nov 26;30(50):7265-70. doi: 10.1016/j.vaccine.2012.09.058. Epub 2012 Oct 2. Vaccine. 2012. PMID: 23041546 Free PMC article.
-
A 30-year journey of trial and error towards a tolerogenic AIDS vaccine.Arch Virol. 2018 Aug;163(8):2025-2031. doi: 10.1007/s00705-018-3936-1. Epub 2018 Jul 24. Arch Virol. 2018. PMID: 30043201 Free PMC article. Review.
-
Aged Chinese-origin rhesus macaques infected with SIV develop marked viremia in absence of clinical disease, inflammation or cognitive impairment.Retrovirology. 2018 Feb 1;15(1):17. doi: 10.1186/s12977-018-0400-y. Retrovirology. 2018. PMID: 29391069 Free PMC article.
References
-
- Fauci AS, Johnston MI, Dieffenbach CW, Burton DR, Hammer SM, et al. HIV vaccine research: the way forward. Science. 2008;321:530–532. - PubMed
-
- Li J, Lord CI, Haseltine W, Letvin NL, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J Acquir Immune Defic Syndr. 1992;5:639–646. - PubMed
-
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. - PubMed
-
- Robb ML. Failure of the Merck HIV vaccine: an uncertain step forward. Lancet. 2008;372:1857–1858. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

