Age-related effects of fasting on ketone body production during lipolysis in rats
- PMID: 21850422
- PMCID: PMC3342632
- DOI: 10.1007/s12199-011-0231-0
Age-related effects of fasting on ketone body production during lipolysis in rats
Abstract
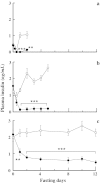
Objective: The age-related effects of fasting on lipolysis, the production of ketone bodies, and plasma insulin levels were studied in male 3-, 8-, and 32-week-old Sprague-Dawley rats.
Methods: The rats were divided into fasting and control groups. The 3-, 8- and 32-week-old rats tolerated fasting for 2, 5, and 12 days, respectively.
Results: Fasting markedly reduced the weights of perirenal and periepididymal white adipose tissues in rats in the three age groups. The mean rates of reduction in both these adipose tissue weights during fasting periods were higher in the order of 3 > 8 > 32-week-old rats. Fasting transiently increased plasma free fatty acid (FFA), total ketone body, β-hydroxybutyrate, and acetoacetate concentrations in the rats in the three age groups. However, plasma FFA, total ketone body, β-hydroxybutyrate, and acetoacetate concentrations in the 3-week-old rats reached maximal peak within 2 days after the onset of fasting, although these concentrations in the 8- and 32-week-old rats took more than 2 days to reach the maximal peak. By contrast, the augmentation of plasma FFA, total ketone body, β-hydroxybutyrate, and acetoacetate concentrations in the rats in the three age groups had declined at the end of each experimental period. Thus, the capacity for fat mobilization was associated with tolerance to fasting. Plasma insulin concentrations in the rats in the three age groups were dramatically reduced during fasting periods, although basal levels of insulin were higher in the order of 32 > 8 > 3 week-old rats.
Conclusion: These results suggest that differences in fat metabolism patterns among rats in the three age groups during prolonged fasting were partly reflected the metabolic turnover rates, plasma insulin levels, and amounts of fat storage.
Figures





References
-
- Williamson DH, Whitelaw E. Physiological aspects of the regulation of ketogenesis. Biochem Soc Symp. 1978;43:137–161. - PubMed
-
- Beylot M. Regulation of in vivo ketogenesis: role of free fatty acids and control by epinephrine, thyroid hormones, insulin and glucagons. Diabetes Metab. 1996;22:299–304. - PubMed
-
- Kalderon B, Mayorek N, Berry E, Zevit N, Bar-Tana J. Fatty acid cycling in the fasting rat. Am J Physiol Endocrinol Metab. 2000;279:E221–E227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
