Membrane attack by complement: the assembly and biology of terminal complement complexes
- PMID: 21850539
- PMCID: PMC3732183
- DOI: 10.1007/s12026-011-8239-5
Membrane attack by complement: the assembly and biology of terminal complement complexes
Abstract
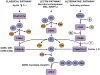
Complement system activation plays an important role in both innate and acquired immunity. Activation of the complement and the subsequent formation of C5b-9 channels (the membrane attack complex) on the cell membranes lead to cell death. However, when the number of channels assembled on the surface of nucleated cells is limited, sublytic C5b-9 can induce cell cycle progression by activating signal transduction pathways and transcription factors and inhibiting apoptosis. This induction by C5b-9 is dependent upon the activation of the phosphatidylinositol 3-kinase/Akt/FOXO1 and ERK1 pathways in a Gi protein-dependent manner. C5b-9 induces sequential activation of CDK4 and CDK2, enabling the G1/S-phase transition and cellular proliferation. In addition, it induces RGC-32, a novel gene that plays a role in cell cycle activation by interacting with Akt and the cyclin B1-CDC2 complex. C5b-9 also inhibits apoptosis by inducing the phosphorylation of Bad and blocking the activation of FLIP, caspase-8, and Bid cleavage. Thus, sublytic C5b-9 plays an important role in cell activation, proliferation, and differentiation, thereby contributing to the maintenance of cell and tissue homeostasis.
Figures



References
-
- Ehrlich P, Sachs H. Ueber die Vielheit der Complemente des Serums. Berliner Klinische Wochenschrift. 1902;14:297–338.
-
- Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. - PubMed
-
- Walport MJ. Complement First of two parts. N Engl J Med. 2001;344:1058–1066. - PubMed
-
- Hugli TE. Biochemistry and biology of anaphylatoxins. Complement. 1986;3:111–127. - PubMed
-
- Frank MM. Complement disorders and hereditary angioedema. J Allergy Clin Immunol. 2010;125:S262–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

