Roles for oestrogen receptor β in adult brain function
- PMID: 21851428
- PMCID: PMC3348521
- DOI: 10.1111/j.1365-2826.2011.02206.x
Roles for oestrogen receptor β in adult brain function
Abstract
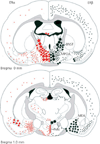
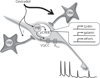
Oestradiol exerts a profound influence upon multiple brain circuits. For the most part, these effects are mediated by oestrogen receptor (ER)α. We review here the roles of ERβ, the other ER isoform, in mediating rodent oestradiol-regulated anxiety, aggressive and sexual behaviours, the control of gonadotrophin secretion, and adult neurogenesis. Evidence exists for: (i) ERβ located in the paraventricular nucleus underpinning the suppressive influence of oestradiol on the stress axis and anxiety-like behaviour; (ii) ERβ expressed in gonadotrophin-releasing hormone neurones contributing to oestrogen negative-feedback control of gonadotrophin secretion; (iii) ERβ controlling the offset of lordosis behaviour; (iv) ERβ suppressing aggressive behaviour in males; (v) ERβ modulating responses to social stimuli; and (vi) ERβ in controlling adult neurogenesis. This review highlights two major themes; first, ERβ and ERα are usually tightly inter-related in the oestradiol-dependent control of a particular brain function. For example, even though oestradiol feedback to control reproduction occurs principally through ERα-dependent mechanisms, modulatory roles for ERβ also exist. Second, the roles of ERα and ERβ within a particular neural network may be synergistic or antagonistic. Examples of the latter include the role of ERα to enhance, and ERβ to suppress, anxiety-like and aggressive behaviours. Splice variants such as ERβ2, acting as dominant negative receptors, are of further particular interest because their expression levels may reflect preceeding oestradiol exposure of relevance to oestradiol replacement therapy. Together, this review highlights the predominant modulatory, but nonetheless important, roles of ERβ in mediating the many effects of oestradiol upon adult brain function.
© 2011 The Authors. Journal of Neuroendocrinology © 2011 Blackwell Publishing Ltd.
Figures


References
-
- Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. - PubMed
-
- Robinson-Rechavi M, Escriva Garcia H, Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116:585–586. - PubMed
-
- Poola I, Abraham J, Baldwin K. Identification of ten exon deleted ERbeta mRNAs in human ovary, breast, uterus and bone tissues: alternate splicing pattern of estrogen receptor beta mRNA is distinct from that of estrogen receptor alpha. FEBS Lett. 2002;516:133–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

