Protocol for minimizing the risk of metachronous adenomas of the colorectum with green tea extract (MIRACLE): a randomised controlled trial of green tea extract versus placebo for nutriprevention of metachronous colon adenomas in the elderly population
- PMID: 21851602
- PMCID: PMC3176243
- DOI: 10.1186/1471-2407-11-360
Protocol for minimizing the risk of metachronous adenomas of the colorectum with green tea extract (MIRACLE): a randomised controlled trial of green tea extract versus placebo for nutriprevention of metachronous colon adenomas in the elderly population
Abstract
Background: Prevention of colorectal cancer is a major health care issue. People who have undergone colonoscopy screening and had colorectal polyps removed have a higher risk of being diagnosed with polyps again compared to the normal population. Therefore, it would be ideal to find appropriate means that effectively help to prevent the reoccurrence of polyps after polypectomy. So far, pharmaceutical chemoprevention with NSAIDs including aspirin has been shown to be effective but not gained general acceptance due to side effects. Nutraceuticals such as polyphenols from tea plants have demonstrated remarkable therapeutic and preventive effects in molecular, epidemiological and clinical trials. However, placebo-controlled trials demonstrating the efficacy of nutraceuticals for the (secondary) prevention of colorectal polyps as precursors for colorectal cancer are missing.
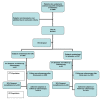
Methods/design: We present the design of a randomized, placebo controlled, multicentre trial to investigate the effect of diet supplementation with green tea extract containing 300 mg epigallocatechin gallate (EGCG), the major polyphenol in green tea, on the recurrence of colon adenomas. Patients who have undergone polypectomy for colonic polyps will be randomized to receive either green tea extract containing 150 mg EGCG two times daily or a placebo over the course of three years. After a one month run-in period in which all patients will receive the active intervention, 2534 patients will be randomized, and 2028 patients are expected to complete the whole study course. Incidence, number and histology of adenoma at endpoint colonoscopy at three years will be compared in both groups.
Discussion: The beneficial safety profile of decaffeinated green tea extract, the quantifiable and known active content EGCG, and the accumulating evidence of its cancer preventive potential require, in our view, a validation of this compound for the nutriprevention of colorectal adenoma. Good accessibility and low costs might render this neutraceutical a top candidate for wider use as food supplement in colon cancer prevention.
Trial registration: ClinicalTrials.gov: NCT01360320.
Figures
References
-
- Cancer WCRFAIf; Research. Food, Nutrition, and Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007.
-
- Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea--a review. J Am Coll Nutr. 2006;25(2):79–99. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

