Anandamide inhibits Theiler's virus induced VCAM-1 in brain endothelial cells and reduces leukocyte transmigration in a model of blood brain barrier by activation of CB(1) receptors
- PMID: 21851608
- PMCID: PMC3173342
- DOI: 10.1186/1742-2094-8-102
Anandamide inhibits Theiler's virus induced VCAM-1 in brain endothelial cells and reduces leukocyte transmigration in a model of blood brain barrier by activation of CB(1) receptors
Abstract
Background: VCAM-1 represents one of the most important adhesion molecule involved in the transmigration of blood leukocytes across the blood-brain barrier (BBB) that is an essential step in the pathogenesis of MS. Several evidences have suggested the potential therapeutic value of cannabinoids (CBs) in the treatment of MS and their experimental models. However, the effects of endocannabinoids on VCAM-1 regulation are poorly understood. In the present study we investigated the effects of anandamide (AEA) in the regulation of VCAM-1 expression induced by Theiler's virus (TMEV) infection of brain endothelial cells using in vitro and in vivo approaches.
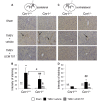
Methods: i) in vitro: VCAM-1 was measured by ELISA in supernatants of brain endothelial cells infected with TMEV and subjected to AEA and/or cannabinoid receptors antagonist treatment. To evaluate the functional effect of VCAM-1 modulation we developed a blood brain barrier model based on a system of astrocytes and brain endothelial cells co-culture. ii) in vivo: CB(1) receptor deficient mice (Cnr1(-/-)) infected with TMEV were treated with the AEA uptake inhibitor UCM-707 for three days. VCAM-1 expression and microglial reactivity were evaluated by immunohistochemistry.
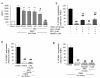
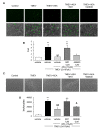
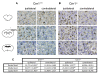
Results: Anandamide-induced inhibition of VCAM-1 expression in brain endothelial cell cultures was mediated by activation of CB(1) receptors. The study of leukocyte transmigration confirmed the functional relevance of VCAM-1 inhibition by AEA. In vivo approaches also showed that the inhibition of AEA uptake reduced the expression of brain VCAM-1 in response to TMEV infection. Although a decreased expression of VCAM-1 by UCM-707 was observed in both, wild type and CB(1) receptor deficient mice (Cnr1(-/-)), the magnitude of VCAM-1 inhibition was significantly higher in the wild type mice. Interestingly, Cnr1(-/-) mice showed enhanced microglial reactivity and VCAM-1 expression following TMEV infection, indicating that the lack of CB(1) receptor exacerbated neuroinflammation.
Conclusions: Our results suggest that CB(1) receptor dependent VCAM-1 inhibition is a novel mechanism for AEA-reduced leukocyte transmigration and contribute to a better understanding of the mechanisms underlying the beneficial role of endocannabinoid system in the Theiler's virus model of MS.
Figures
References
-
- Brosnan CF, Cannella B, Battistini L, Raine CS. Cytokine localization in multiple sclerosis lesions: correlation with adhesion molecule expression and reactive nitrogen species. Neurology. 1995;45:S16–S21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

