Hybrid magnetic nanostructures (MNS) for magnetic resonance imaging applications
- PMID: 21851844
- PMCID: PMC3381436
- DOI: 10.1016/j.addr.2011.07.001
Hybrid magnetic nanostructures (MNS) for magnetic resonance imaging applications
Abstract
The development of MRI contrast agents has experienced its version of the gilded age over the past decade, thanks largely to the rapid advances in nanotechnology. In addition to progress in single mode contrast agents, which ushered in unprecedented R(1) or R(2) sensitivities, there has also been a boon in the development of agents covering more than one mode of detection. These include T(1)-PET, T(2)-PET T(1)-optical, T(2)-optical, T(1)-T(2) agents and many others. In this review, we describe four areas which we feel have experienced particular growth due to nanotechnology, specifically T(2) magnetic nanostructure development, T(1)/T(2)-optical dual mode agents, and most recently the T(1)-T(2) hybrid imaging systems. In each of these systems, we describe applications including in vitro, in vivo usage and assay development. In all, while the benefits and drawbacks of most MRI contrast agents depend on the application at hand, the recent development in multimodal nanohybrids may curtail the shortcomings of single mode agents in diagnostic and clinical settings by synergistically incorporating functionality. It is hoped that as nanotechnology advances over the next decade, it will produce agents with increased diagnostics and assay relevant capabilities in streamlined packages that can meaningfully improve patient care and prognostics. In this review article, we focus on T(2) materials, its surface functionalization and coupling with optical and/or T(1) agents.
Published by Elsevier B.V.
Figures

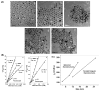
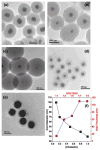


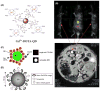



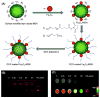


References
-
- Brenner DJ, Hall EJ. Current concepts - Computed tomography - An increasing source of radiation exposure. New England Journal of Medicine. 2007;357:2277–2284. - PubMed
-
- Basu S, Chryssikos T, Moghadam-Kia S, Zhuang HM, Torigian DA, Alavi A. Positron Emission Tomography as a Diagnostic Tool in Infection: Present Role and Future Possibilities. Seminars in Nuclear Medicine. 2009;39:36–51. - PubMed
-
- Meerwaldt R, Slart RHJA, van Dam GM, Luijckx GJ, Tio RA, Zeebregts CJ. PET/SPECT imaging: From carotid vulnerability to brain viability. European Journal of Radiology. 2010;74:104–109. - PubMed
-
- Hong R, Fischer NO, Emrick T, Rotello VM. Surface PEGylation and Ligand Exchange Chemistry of FePt Nanoparticles for Biological Applications. Chemistry of Materials. 2005;17:4617–4621.
-
- Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nature Reviews Neuroscience. 2002;3:142–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

