The transferrin receptor and the targeted delivery of therapeutic agents against cancer
- PMID: 21851850
- PMCID: PMC3500658
- DOI: 10.1016/j.bbagen.2011.07.016
The transferrin receptor and the targeted delivery of therapeutic agents against cancer
Abstract
Background: Traditional cancer therapy can be successful in destroying tumors, but can also cause dangerous side effects. Therefore, many targeted therapies are in development. The transferrin receptor (TfR) functions in cellular iron uptake through its interaction with transferrin. This receptor is an attractive molecule for the targeted therapy of cancer since it is upregulated on the surface of many cancer types and is efficiently internalized. This receptor can be targeted in two ways: 1) for the delivery of therapeutic molecules into malignant cells or 2) to block the natural function of the receptor leading directly to cancer cell death.
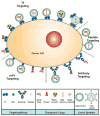
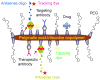
Scope of review: In the present article we discuss the strategies used to target the TfR for the delivery of therapeutic agents into cancer cells. We provide a summary of the vast types of anti-cancer drugs that have been delivered into cancer cells employing a variety of receptor binding molecules including Tf, anti-TfR antibodies, or TfR-binding peptides alone or in combination with carrier molecules including nanoparticles and viruses.
Major conclusions: Targeting the TfR has been shown to be effective in delivering many different therapeutic agents and causing cytotoxic effects in cancer cells in vitro and in vivo.
General significance: The extensive use of TfR for targeted therapy attests to the versatility of targeting this receptor for therapeutic purposes against malignant cells. More advances in this area are expected to further improve the therapeutic potential of targeting the TfR for cancer therapy leading to an increase in the number of clinical trials of molecules targeting this receptor. This article is part of a Special Issue entitled Transferrins: molecular mechanisms of iron transport and disorders.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


References
-
- Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121:144–158. - PubMed
-
- Cheng Y, Zak O, Aisen P, Harrison SC, Walz T. Structure of the human transferrin receptor-transferrin complex. Cell. 2004;116:565–576. - PubMed
-
- Fuchs H, Lucken U, Tauber R, Engel A, Gessner R. Structural model of phospholipid-reconstituted human transferrin receptor derived by electron microscopy. Structure. 1998;6:1235–1243. - PubMed
-
- Lebron JA, Bennett MJ, Vaughn DE, Chirino AJ, Snow PM, Mintier GA, Feder JN, Bjorkman PJ. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell. 1998;93:111–123. - PubMed
-
- Hemadi M, Kahn PH, Miquel G, El Hage Chahine JM. Transferrin’s mechanism of interaction with receptor 1. Biochemistry. 2004;43:1736–1745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

