A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells
- PMID: 21851965
- PMCID: PMC3185176
- DOI: 10.1016/j.scitotenv.2011.07.039
A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells
Abstract
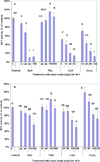
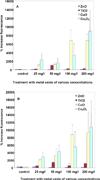
Nanoparticles (NPs), including nanometal oxides, are being used in diverse applications such as medicine, clothing, cosmetics and food. In order to promote the safe development of nanotechnology, it is essential to assess the potential adverse health consequences associated with human exposure. The liver is a target site for NP toxicity, due to NP accumulation within it after ingestion, inhalation or absorption. The toxicity of nano-ZnO, TiO(2), CuO and Co(3)O(4) was investigated using a primary culture of channel catfish hepatocytes and human HepG2 cells as in vitro model systems for assessing the impact of metal oxide NPs on human and environmental health. Some mechanisms of nanotoxicity were determined by using phase contrast inverted microscopy, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, reactive oxygen species (ROS) assays, and flow cytometric assays. Nano-CuO and ZnO showed significant toxicity in both HepG2 cells and catfish primary hepatocytes. The results demonstrate that HepG2 cells are more sensitive than catfish primary hepatocytes to the toxicity of metal oxide NPs. The overall ranking of the toxicity of metal oxides to the test cells is as follows: TiO(2)<Co(3)O(4)<ZnO<CuO. The toxicity is due not only to ROS-induced cell death, but also to damages to cell and mitochondrial membranes.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures





Similar articles
-
Size-dependent toxicity of metal oxide particles--a comparison between nano- and micrometer size.Toxicol Lett. 2009 Jul 24;188(2):112-8. doi: 10.1016/j.toxlet.2009.03.014. Epub 2009 Mar 26. Toxicol Lett. 2009. PMID: 19446243
-
A practical approach to assess inhalation toxicity of metal oxide nanoparticles in vitro.J Appl Toxicol. 2018 Feb;38(2):160-171. doi: 10.1002/jat.3518. Epub 2017 Sep 28. J Appl Toxicol. 2018. PMID: 28960351
-
3-Hydroxyflavone enhances the toxicity of ZnO nanoparticles in vitro.J Appl Toxicol. 2018 Sep;38(9):1206-1214. doi: 10.1002/jat.3633. Epub 2018 Apr 25. J Appl Toxicol. 2018. PMID: 29691881
-
Research progress on toxicity, function, and mechanism of metal oxide nanoparticles on vascular endothelial cells.J Appl Toxicol. 2021 May;41(5):683-700. doi: 10.1002/jat.4121. Epub 2020 Nov 26. J Appl Toxicol. 2021. PMID: 33244813 Review.
-
Copper oxide nanoparticles: In vitro and in vivo toxicity, mechanisms of action and factors influencing their toxicology.Comp Biochem Physiol C Toxicol Pharmacol. 2023 Sep;271:109682. doi: 10.1016/j.cbpc.2023.109682. Epub 2023 Jun 15. Comp Biochem Physiol C Toxicol Pharmacol. 2023. PMID: 37328134 Review.
Cited by
-
In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells.Nanoscale Res Lett. 2013 Nov 21;8(1):496. doi: 10.1186/1556-276X-8-496. Nanoscale Res Lett. 2013. PMID: 24261419 Free PMC article.
-
Assessing the effect of β-glucan diets on innate immune response of tilapia macrophages against trichlorfon exposure: an in vitro study.Fish Physiol Biochem. 2024 Apr;50(2):527-541. doi: 10.1007/s10695-023-01283-5. Epub 2023 Dec 15. Fish Physiol Biochem. 2024. PMID: 38099984 Free PMC article.
-
Updates on Biogenic Metallic and Metal Oxide Nanoparticles: Therapy, Drug Delivery and Cytotoxicity.Pharmaceutics. 2023 Jun 3;15(6):1650. doi: 10.3390/pharmaceutics15061650. Pharmaceutics. 2023. PMID: 37376098 Free PMC article. Review.
-
CuO-NPs Induce Apoptosis and Functional Impairment in BV2 Cells Through the CSF-1R/PLCγ2/ERK/Nrf2 Pathway.Toxics. 2025 Mar 21;13(4):231. doi: 10.3390/toxics13040231. Toxics. 2025. PMID: 40278547 Free PMC article.
-
The effect of ZnO nanoparticles on liver function in rats.Int J Nanomedicine. 2016 Aug 31;11:4275-85. doi: 10.2147/IJN.S109031. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27621621 Free PMC article.
References
-
- Adams LK, Lyon DY, Alvarez PJJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40:3527–3532. - PubMed
-
- Aker WG, Hu XK, Wang P, Hwang HM. Comparing the relative toxicity of malathion and malaoxon in blue catfish Ictalurus furcatus. Environ Toxicol. 2008;23:548–554. - PubMed
-
- Almofti MR, Ichikawa T, Yamashita K, Terada H, Shinohara Y. Silver ion induces acyclosporine a-sensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome c. J Biochem. 2003;134:43–49. - PubMed
-
- Becheri A, Dürr M, Nostro PL, Baglioni P. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J Nanopart Res. 2008;10:679–689.
-
- Boess F, Kamber M, Romer S, Gasser R, Muller D, Albertini S, Suter L. Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: possible implications for toxicogenomics use of in vitro systems. Toxicol Sci. 2003;73:386–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

