Cell cycle-regulated multi-site phosphorylation of Neurogenin 2 coordinates cell cycling with differentiation during neurogenesis
- PMID: 21852393
- PMCID: PMC3171226
- DOI: 10.1242/dev.067900
Cell cycle-regulated multi-site phosphorylation of Neurogenin 2 coordinates cell cycling with differentiation during neurogenesis
Abstract
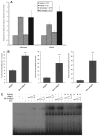
During development of the central nervous system, the transition from progenitor maintenance to differentiation is directly triggered by a lengthening of the cell cycle that occurs as development progresses. However, the mechanistic basis of this regulation is unknown. The proneural transcription factor Neurogenin 2 (Ngn2) acts as a master regulator of neuronal differentiation. Here, we demonstrate that Ngn2 is phosphorylated on multiple serine-proline sites in response to rising cyclin-dependent kinase (cdk) levels. This multi-site phosphorylation results in quantitative inhibition of the ability of Ngn2 to induce neurogenesis in vivo and in vitro. Mechanistically, multi-site phosphorylation inhibits binding of Ngn2 to E box DNA, and inhibition of DNA binding depends on the number of phosphorylation sites available, quantitatively controlling promoter occupancy in a rheostat-like manner. Neuronal differentiation driven by a mutant of Ngn2 that cannot be phosphorylated by cdks is no longer inhibited by elevated cdk kinase levels. Additionally, phosphomutant Ngn2-driven neuronal differentiation shows a reduced requirement for the presence of cdk inhibitors. From these results, we propose a model whereby multi-site cdk-dependent phosphorylation of Ngn2 interprets cdk levels to control neuronal differentiation in response to cell cycle lengthening during development.
Figures




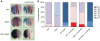


References
-
- Aguado-Llera D., Goormaghtigh E., de Geest N., Quan X. J., Prieto A., Hassan B. A., Gomez J., Neira J. L. (2010). The basic helix-loop-helix region of human neurogenin 1 is a monomeric natively unfolded protein which forms a ``fuzzy'' complex upon DNA binding. Biochemistry 49, 1577-1589 - PubMed
-
- Bertrand N., Castro D. S., Guillemot F. (2002). Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517-530 - PubMed
-
- Crane-Robinson C., Dragan A. I., Privalov P. L. (2006). The extended arms of DNA-binding domains: a tale of tails. Trends Biochem. Sci. 31, 547-552 - PubMed
-
- Cremisi F., Philpott A., Ohnuma S. (2003). Cell cycle and cell fate interactions in neural development. Curr. Opin. Neurobiol. 13, 26-33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

