Efficacy of influenza A H1N1/2009 vaccine in hemodialysis and kidney transplant patients
- PMID: 21852661
- PMCID: PMC3358996
- DOI: 10.2215/CJN.02160311
Efficacy of influenza A H1N1/2009 vaccine in hemodialysis and kidney transplant patients
Abstract
Background and objectives: Data are needed to assess safety and efficacy of the 2009 pandemic influenza A H1N1 vaccine in renal patients.
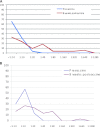
Design, setting, participants, & measurements: We prospectively evaluated seroconversion, predictors of response, and vaccine safety in renal patients. Hemagglutination inhibition tests to detect serum antibodies against a new influenza A-H1N1 virus were performed in 79 transplant patients, 48 hemodialysis patients, and 15 healthy workers before and 1 month after vaccination. Healthy controls and 88 of 127 renal patients were vaccinated. Seroconversion was defined as at least 2 dilutions increase in titer.
Results: We excluded 19 individuals seroprotected (≥1/40) against the novel H1N1 in the initial sample. Efficacy rate in the 96 vaccinated individuals was 43.7% (42 of 96 seroconverted versus four of 27 nonvaccinated patients, P = 0.007). For vaccinated subgroups, efficacy was 41.8% in transplant patients (P = 0.039 versus nonvaccinated), 33.3% in hemodialysis patients (P = 0.450), and 81.8% in controls. Healthy controls showed better response to vaccine than transplant (P = 0.021) and dialysis (P = 0.012) patients. For the transplant subgroup, longer time after transplantation (P = 0.028) was associated with seroconversion, but no influence was found for age, gender, renal function, or immunosuppression. In the hemodialysis subgroup, younger age was associated with response (55.7 ± 20.8 versus 71.6 ± 10.1 years, P = 0.042), but other specific variables, including Kt/V or time on dialysis, were not. No serious adverse events were reported, and kidney function was stable.
Conclusion: The novel influenza A 2009 H1N1 vaccine was safe in renal patients, although administration of a single dose of adjuvanted vaccine induced a poor response in these patients.
Figures

Comment in
-
Vaccinations in kidney transplant patients: searching for optimal protection.Clin J Am Soc Nephrol. 2011 Sep;6(9):2099-101. doi: 10.2215/CJN.07330711. Epub 2011 Aug 18. Clin J Am Soc Nephrol. 2011. PMID: 21852666 No abstract available.
References
-
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Emergence of a novel swine-origin influenza A (H1N1) in humans. N Engl J Med 360:2602–2615, 2009 - PubMed
-
- New influenza A/H1N1 virus: Global epidemiological situation. Wkly Epidemiol Rec 84: 249–257, 2009 - PubMed
-
- Kumar D, Michaels MG, Morris MI, Green M, Avery RK, Liu C, Danziger-Isakov L, Stosor V, Estabrook M, Gantt S, Marr KA, Martin S, Silveira FP, Razonable RR, Allen UD, Levi ME, Lyon GM, Bell LE, Huprikar S, Patel G, Gregg KS, Pursell K, Helmersen D, Julian KG, Shiley K, Bono B, Dharnidharka VR, Alavi G, Kalpoe JS, Shoham S, Reid GE, Humar A, American Society of Transplantation H1N1 Collaborative Study Group: Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: A multicentre cohort study. Lancet Infect Dis 10: 521–526, 2010 - PMC - PubMed
-
- Ridao-Cano N, Sanchez-Fructuoso AI, Rodriguez-Moreno A, Barrientos A: H1N1 2009 Influenza in kidney transplant patients. Transplantation 90: 224–225, 2010 - PubMed
-
- Vajo Z, Tamas F, Sinka L, Jankovics I: Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009–10 influenza season: A multicentre, randomised controlled trial. Lancet 375: 49–55, 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

