GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice
- PMID: 21852673
- PMCID: PMC3178286
- DOI: 10.2337/db10-1740
GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice
Abstract
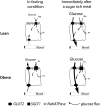
Objective: In healthy rodents, intestinal sugar absorption in response to sugar-rich meals and insulin is regulated by GLUT2 in enterocyte plasma membranes. Loss of insulin action maintains apical GLUT2 location. In human enterocytes, apical GLUT2 location has not been reported but may be revealed under conditions of insulin resistance.
Research design and methods: Subcellular location of GLUT2 in jejunal enterocytes was analyzed by confocal and electron microscopy imaging and Western blot in 62 well-phenotyped morbidly obese subjects and 7 lean human subjects. GLUT2 locations were assayed in ob/ob and ob/+ mice receiving oral metformin or in high-fat low-carbohydrate diet-fed C57Bl/6 mice. Glucose absorption and secretion were respectively estimated by oral glucose tolerance test and secretion of [U-(14)C]-3-O-methyl glucose into lumen.
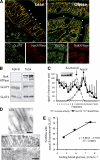
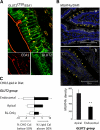
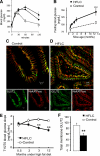
Results: In human enterocytes, GLUT2 was consistently located in basolateral membranes. Apical GLUT2 location was absent in lean subjects but was observed in 76% of obese subjects and correlated with insulin resistance and glycemia. In addition, intracellular accumulation of GLUT2 with early endosome antigen 1 (EEA1) was associated with reduced MGAT4a activity (glycosylation) in 39% of obese subjects on a low-carbohydrate/high-fat diet. Mice on a low-carbohydrate/high-fat diet for 12 months also exhibited endosomal GLUT2 accumulation and reduced glucose absorption. In ob/ob mice, metformin promoted apical GLUT2 and improved glucose homeostasis. Apical GLUT2 in fasting hyperglycemic ob/ob mice tripled glucose release into intestinal lumen.
Conclusions: In morbidly obese insulin-resistant subjects, GLUT2 was accumulated in apical and/or endosomal membranes of enterocytes. Functionally, apical GLUT2 favored and endosomal GLUT2 reduced glucose transepithelial exchanges. Thus, altered GLUT2 locations in enterocytes are a sign of intestinal adaptations to human metabolic pathology.
Figures

References
-
- Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med 2007;261:32–43 - PubMed
-
- Chaikomin R, Doran S, Jones KL, et al. Initially more rapid small intestinal glucose delivery increases plasma insulin, GIP, and GLP-1 but does not improve overall glycemia in healthy subjects. Am J Physiol Endocrinol Metab 2005;289:E504–E507 - PubMed
-
- Raybould HE. Nutrient sensing in the gastrointestinal tract: possible role for nutrient transporters. J Physiol Biochem 2008;64:349–356 - PubMed
-
- Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem 1992;267:14523–14526 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

