HIV diagnostic testing: evolving technology and testing strategies
- PMID: 21852712
- PMCID: PMC6148855
HIV diagnostic testing: evolving technology and testing strategies
Abstract
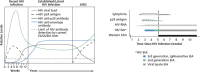
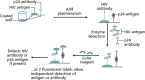
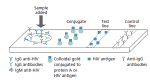
Detection of acute HIV infection is important to public health because this stage is one of high infectiousness and appears to account for a disproportionate amount of HIV transmission. Newer technologies in HIV testing, including third-generation enzyme immunoassays (EIAs) that detect anti-HIV IgM and IgG antibodies, fourth-generation combination EIAs that detect both anti-HIV antibodies and HIV p24 antigen, and nucleic acid-based testing for HIV RNA, have markedly reduced the interval between infection and detection of infection. Rapid diagnostic tests including assays for IgG and IgM anti-HIV antibodies have high sensitivity and specificity. The availability and wide use of these newer technologies have motivated review of recommended HIV testing algorithms. Individuals' knowledge of their HIV serostatus contributes to reducing transmission risk behaviors. Thus, widespread testing, facilitated by newer technology, allows more individuals to know their serostatus and is the first step in any successful effort to curb the incidence of HIV infection. This article summarizes a lecture by Demetre Daskalakis, MD, at the New York City IAS-USA continuing medical education program held in November 2009 and re-presented in December 2010.
Conflict of interest statement
Financial Disclosure: Dr Daskalakis has no relevant financial affiliations to disclose.
Figures
Similar articles
-
Reduction of the diagnostic window in three cases of human immunodeficiency-1 subtype E primary infection with fourth-generation HIV screening assays.Vox Sang. 2003 Aug;85(2):73-9. doi: 10.1046/j.1423-0410.2003.00334.x. Vox Sang. 2003. PMID: 12925157
-
Detection of acute HIV infection: we can't close the window.J Infect Dis. 2012 Feb 15;205(4):521-4. doi: 10.1093/infdis/jir793. Epub 2011 Dec 29. J Infect Dis. 2012. PMID: 22207652 No abstract available.
-
Fourth-generation enzyme immunoassays for screening of HIV in blood donors: need of the hour.Indian J Pathol Microbiol. 2011 Apr-Jun;54(2):433-4. doi: 10.4103/0377-4929.81623. Indian J Pathol Microbiol. 2011. PMID: 21623129 No abstract available.
-
Measurement of HIV-1 p24 antigen by signal-amplification-boosted ELISA of heat-denatured plasma is a simple and inexpensive alternative to tests for viral RNA.AIDS Rev. 2002 Apr-Jun;4(2):83-92. AIDS Rev. 2002. PMID: 12152521 Review.
-
More reliable diagnosis of infection with human immunodeficiency virus type 1 (HIV-1) by detection of antibody IgGs to pol and gag proteins of HIV-1 and p24 antigen of HIV-1 in urine, saliva, and/or serum with highly sensitive and specific enzyme immunoassay (immune complex transfer enzyme immunoassay): a review.J Clin Lab Anal. 1997;11(5):267-86. doi: 10.1002/(SICI)1098-2825(1997)11:5<267::AID-JCLA5>3.0.CO;2-4. J Clin Lab Anal. 1997. PMID: 9292394 Free PMC article. Review.
Cited by
-
Impact of naturally occurring amino acid variations on the detection of HIV-1 p24 in diagnostic antigen tests.BMC Infect Dis. 2015 Oct 29;15:468. doi: 10.1186/s12879-015-1174-7. BMC Infect Dis. 2015. PMID: 26511217 Free PMC article.
-
HIV prevention and missed opportunities among people with recently acquired HIV infection: Α protocol for a systematic review.PLoS One. 2024 Dec 31;19(12):e0295462. doi: 10.1371/journal.pone.0295462. eCollection 2024. PLoS One. 2024. PMID: 39739779 Free PMC article.
-
HIV testing: the cornerstone of HIV prevention efforts in the USA.Future Virol. 2011 Nov;6(11):10.2217/fvl.11.114. doi: 10.2217/fvl.11.114. Future Virol. 2011. PMID: 37965646 Free PMC article.
-
A cocktail of thermally stable, chemically synthesized capture agents for the efficient detection of anti-gp41 antibodies from human sera.PLoS One. 2013 Oct 7;8(10):e76224. doi: 10.1371/journal.pone.0076224. eCollection 2013. PLoS One. 2013. PMID: 24116098 Free PMC article.
-
Development and evaluation of a high-sensitivity RT-PCR lateral flow assay for early detection of HIV-1 infection.Heliyon. 2024 Jun 12;10(12):e32784. doi: 10.1016/j.heliyon.2024.e32784. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38975074 Free PMC article.
References
-
- Centers for Disease Control (CDC). Interpretation and use of the Western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. MMWR Morb Mortal Wkly Rep. 1989;38(Suppl 7):1-7. - PubMed
-
- Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1-17. - PubMed
-
- Daskalakis D, Landovitz R, Cespedes M. Chapter 11: Sexual health. In: Makadon H, Mayer K, Potter J, Goldhammer H, eds. The Fenway Guide to Lesbian, Gay, Bisexual and Transgender Health. Philadelphia, PA: ACP Press; 2008:286, Figure 11.1.
-
- Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871-1879. - PubMed
-
- Midthun K, Garrison L, Clements ML, Farzadegan H, Fernie B, Quinn T. Frequency of indeterminate Western blot tests in healthy adults at low risk for human immunodeficiency virus infection. The NIAID AIDS Vaccine Clinical Trials Network. J Infect Dis. 1990;162:1379-1382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
