Suppression of scant identifies Endos as a substrate of greatwall kinase and a negative regulator of protein phosphatase 2A in mitosis
- PMID: 21852956
- PMCID: PMC3154957
- DOI: 10.1371/journal.pgen.1002225
Suppression of scant identifies Endos as a substrate of greatwall kinase and a negative regulator of protein phosphatase 2A in mitosis
Abstract
Protein phosphatase 2A (PP2A) plays a major role in dephosphorylating the targets of the major mitotic kinase Cdk1 at mitotic exit, yet how it is regulated in mitotic progression is poorly understood. Here we show that mutations in either the catalytic or regulatory twins/B55 subunit of PP2A act as enhancers of gwl(Scant), a gain-of-function allele of the Greatwall kinase gene that leads to embryonic lethality in Drosophila when the maternal dosage of the mitotic kinase Polo is reduced. We also show that heterozygous mutant endos alleles suppress heterozygous gwl(Scant); many more embryos survive. Furthermore, heterozygous PP2A mutations make females heterozygous for the strong mutation polo(11) partially sterile, even in the absence of gwl(Scant). Heterozygosity for an endos mutation suppresses this PP2A/polo(11) sterility. Homozygous mutation or knockdown of endos leads to phenotypes suggestive of defects in maintaining the mitotic state. In accord with the genetic interactions shown by the gwl(Scant) dominant mutant, the mitotic defects of Endos knockdown in cultured cells can be suppressed by knockdown of either the catalytic or the Twins/B55 regulatory subunits of PP2A but not by the other three regulatory B subunits of Drosophila PP2A. Greatwall phosphorylates Endos at a single site, Ser68, and this is essential for Endos function. Together these interactions suggest that Greatwall and Endos act to promote the inactivation of PP2A-Twins/B55 in Drosophila. We discuss the involvement of Polo kinase in such a regulatory loop.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

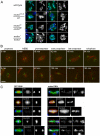
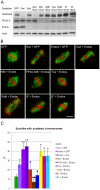

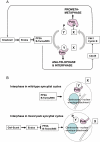
Comment in
-
Polo, greatwall, and protein phosphatase PP2A Jostle for pole position.PLoS Genet. 2011 Aug;7(8):e1002213. doi: 10.1371/journal.pgen.1002213. Epub 2011 Aug 11. PLoS Genet. 2011. PMID: 21852954 Free PMC article. No abstract available.
References
-
- Bettencourt-Dias M, Giet R, Sinka R, Mazumdar A, Lock WG, et al. Genome-wide survey of protein kinases required for cell cycle progression. Nature. 2004;432:980–987. - PubMed
-
- Yu J, Zhao Y, Li Z, Galas S, Goldberg ML. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell. 2006;22:83–91. - PubMed
-
- Archambault V, Zhao X, White-Cooper H, Carpenter ATC, Glover DM. Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with Polo kinase. PLoS Genet. 2007;3:e200. doi: 10.1371/journal.pgen.0030200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

