Cell signaling underlying epileptic behavior
- PMID: 21852968
- PMCID: PMC3151612
- DOI: 10.3389/fnbeh.2011.00045
Cell signaling underlying epileptic behavior
Abstract
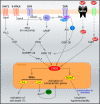
Epilepsy is a complex disease, characterized by the repeated occurrence of bursts of electrical activity (seizures) in specific brain areas. The behavioral outcome of seizure events strongly depends on the brain regions that are affected by overactivity. Here we review the intracellular signaling pathways involved in the generation of seizures in epileptogenic areas. Pathways activated by modulatory neurotransmitters (dopamine, norepinephrine, and serotonin), involving the activation of extracellular-regulated kinases and the induction of immediate early genes (IEGs) will be first discussed in relation to the occurrence of acute seizure events. Activation of IEGs has been proposed to lead to long-term molecular and behavioral responses induced by acute seizures. We also review deleterious consequences of seizure activity, focusing on the contribution of apoptosis-associated signaling pathways to the progression of the disease. A deep understanding of signaling pathways involved in both acute- and long-term responses to seizures continues to be crucial to unravel the origins of epileptic behaviors and ultimately identify novel therapeutic targets for the cure of epilepsy.
Keywords: ERK; Fos; activity-dependent transcription; apoptosis; hippocampus; seizure.
Figures

References
-
- Araujo I. M., Gil J. M., Carreira B. P., Mohapel P., Petersen A., Pinheiro P. S., Soulet D., Bahr B. A., Brundin P., Carvalho C. M. (2008). Calpain activation is involved in early caspase-independent neurodegeneration in the hippocampus following status epilepticus. J. Neurochem. 105, 666–67610.1111/j.1471-4159.2007.05181.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

