Targeted over-expression of glutamate transporter 1 (GLT-1) reduces ischemic brain injury in a rat model of stroke
- PMID: 21853027
- PMCID: PMC3154194
- DOI: 10.1371/journal.pone.0022135
Targeted over-expression of glutamate transporter 1 (GLT-1) reduces ischemic brain injury in a rat model of stroke
Abstract
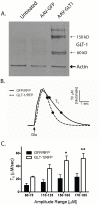
Following the onset of an ischemic brain injury, the excitatory neurotransmitter glutamate is released. The excitotoxic effects of glutamate are a major contributor to the pathogenesis of a stroke. The aim of this study was to examine if overexpression of a glutamate transporter (GLT-1) reduces ischemic brain injury in a rat model of stroke. We generated an adeno-associated viral (AAV) vector expressing the rat GLT-1 cDNA (AAV-GLT1). Functional expression of AAV-GLT1 was confirmed by increased glutamate clearance rate in non-stroke rat brain as measured by in vivo amperometry. AAV-GLT1 was injected into future cortical region of infarction 3 weeks prior to 60 min middle cerebral artery occlusion (MCAo). Tissue damage was assessed at one and two days after MCAo using TUNEL and TTC staining, respectively. Behavioral testing was performed at 2, 8 and 14 days post-stroke. Animals receiving AAV-GLT1, compared to AAV-GFP, showed significant decreases in the duration and magnitude of extracellular glutamate, measured by microdialysis, during the 60 minute MCAo. A significant reduction in brain infarction and DNA fragmentation was observed in the region of AAV-GLT1 injection. Animals that received AAV-GLT1 showed significant improvement in behavioral recovery following stroke compared to the AAV-GFP group. We demonstrate that focal overexpression of the glutamate transporter, GLT-1, significantly reduces ischemia-induced glutamate overflow, decreases cell death and improves behavioral recovery. These data further support the role of glutamate in the pathogenesis of ischemic damage in brain and demonstrate that targeted gene delivery to decrease the ischemia-induced glutamate overflow reduces the cellular and behavioral deficits caused by stroke.
Conflict of interest statement
Figures





References
-
- Martin RL, Lloyd HGE, Cowan AI. The early events of oxygen and glucose deprivation - setting the scene for neuronal death. Trends in Neurosciences. 1994;17:251–257. - PubMed
-
- Garber K. Stroke treatment–light at the end of the tunnel? Nat Biotechnol. 2007;25:838–840. - PubMed
-
- Sheardown MJ, Nielsen EO, Hansen AJ, Jacobsen P, Honore T. 2,3-Dihydroxy-6-Nitro-7-Sulfamoyl-Benzo(F)Quinoxaline - A neuroprotectant for cerebral ischemia. Science. 1990;247:571–574. - PubMed
-
- Simon RP, Swan JH, Griffiths T, Meldrum BS. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984;226:850–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

