A role for glutamate transporters in the regulation of insulin secretion
- PMID: 21853059
- PMCID: PMC3154915
- DOI: 10.1371/journal.pone.0022960
A role for glutamate transporters in the regulation of insulin secretion
Abstract
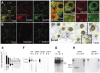
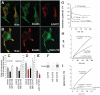
In the brain, glutamate is an extracellular transmitter that mediates cell-to-cell communication. Prior to synaptic release it is pumped into vesicles by vesicular glutamate transporters (VGLUTs). To inactivate glutamate receptor responses after release, glutamate is taken up into glial cells or neurons by excitatory amino acid transporters (EAATs). In the pancreatic islets of Langerhans, glutamate is proposed to act as an intracellular messenger, regulating insulin secretion from β-cells, but the mechanisms involved are unknown. By immunogold cytochemistry we show that insulin containing secretory granules express VGLUT3. Despite the fact that they have a VGLUT, the levels of glutamate in these granules are low, indicating the presence of a protein that can transport glutamate out of the granules. Surprisingly, in β-cells the glutamate transporter EAAT2 is located, not in the plasma membrane as it is in brain cells, but exclusively in insulin-containing secretory granules, together with VGLUT3. In EAAT2 knock out mice, the content of glutamate in secretory granules is higher than in wild type mice. These data imply a glutamate cycle in which glutamate is carried into the granules by VGLUT3 and carried out by EAAT2. Perturbing this cycle by knocking down EAAT2 expression with a small interfering RNA, or by over-expressing EAAT2 or a VGLUT in insulin granules, significantly reduced the rate of granule exocytosis. Simulations of granule energetics suggest that VGLUT3 and EAAT2 may regulate the pH and membrane potential of the granules and thereby regulate insulin secretion. These data suggest that insulin secretion from β-cells is modulated by the flux of glutamate through the secretory granules.
Conflict of interest statement
Figures




References
-
- Ottersen OP, Storm-Mathisen J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol. 1984;229:374–392. - PubMed
-
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. - PubMed
-
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. - PubMed
-
- Balcar V, Johnston GA. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J Neurochem. 1972;19:2657–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

