Early potent protection against heterologous SIVsmE660 challenge following live attenuated SIV vaccination in Mauritian cynomolgus macaques
- PMID: 21853072
- PMCID: PMC3154277
- DOI: 10.1371/journal.pone.0023092
Early potent protection against heterologous SIVsmE660 challenge following live attenuated SIV vaccination in Mauritian cynomolgus macaques
Abstract
Background: Live attenuated simian immunodeficiency virus (SIV) vaccines represent the most effective means of vaccinating macaques against pathogenic SIV challenge. However, thus far, protection has been demonstrated to be more effective against homologous than heterologous strains. Immune correlates of vaccine-induced protection have also been difficult to identify, particularly those measurable in the peripheral circulation.
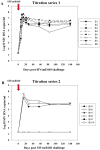
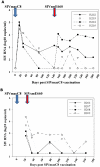
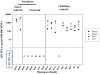
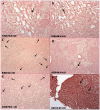
Methodology/principal findings: Here we describe potent protection in 6 out of 8 Mauritian-derived cynomolgus macaques (MCM) against heterologous virus challenge with the pathogenic, uncloned SIVsmE660 viral stock following vaccination with live attenuated SIVmac251/C8. MCM provided a characterised host genetic background with limited Major Histocompatibility Complex (MHC) and TRIM5α allelic diversity. Early protection, observed as soon as 3 weeks post-vaccination, was comparable to that of 20 weeks vaccination. Recrudescence of vaccine virus was most pronounced in breakthrough cases where simultaneous identification of vaccine and challenge viruses by virus-specific PCR was indicative of active co-infection. Persistence of the vaccine virus in a range of lymphoid tissues was typified by a consistent level of SIV RNA positive cells in protected vaccinates. However, no association between MHC class I/II haplotype or TRIM5α polymorphism and study outcome was identified.
Conclusion/significance: This SIV vaccine study, conducted in MHC-characterised MCM, demonstrated potent protection against the pathogenic, heterologous SIVsmE660 challenge stock after only 3 weeks vaccination. This level of protection against this viral stock by intravenous challenge has not been hitherto observed. The mechanism(s) of protection by vaccination with live attenuated SIV must account for the heterologous and early protection data described in this study, including those which relate to the innate immune system.
Conflict of interest statement
Figures






References
-
- Gaschen B, Taylor J, Yusim K, Foley B, Gao F, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–2360. - PubMed
-
- McCutchan FE. Global epidemiology of HIV. J Med Virol. 2006;78:S7–S12. - PubMed
-
- Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, et al. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006;7:19–23. - PubMed
-
- Abel K, Compton L, Rourke T, Montefiori D, Lu D, et al. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol. 2003;77:3099–3118. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

