Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104
- PMID: 21853076
- PMCID: PMC3154919
- DOI: 10.1371/journal.pone.0023108
Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104
Abstract
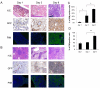
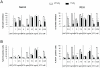
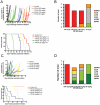
Recent studies indicate that interactions between leukemia cells and the bone marrow (BM) microenvironment promote leukemia cell survival and confer resistance to anti-leukemic drugs. There is evidence that BM microenvironment contains hypoxic areas that confer survival advantage to hematopoietic cells. In the present study we investigated whether hypoxia in leukemic BM contributes to the protective role of the BM microenvironment. We observed a marked expansion of hypoxic BM areas in immunodeficient mice engrafted with acute lymphoblastic leukemia (ALL) cells. Consistent with this finding, we found that hypoxia promotes chemoresistance in various ALL derived cell lines. These findings suggest to employ hypoxia-activated prodrugs to eliminate leukemia cells within hypoxic niches. Using several xenograft models, we demonstrated that administration of the hypoxia-activated dinitrobenzamide mustard, PR-104 prolonged survival and decreased leukemia burden of immune-deficient mice injected with primary acute lymphoblastic leukemia cells. Together, these findings strongly suggest that targeting hypoxia in leukemic BM is feasible and may significantly improve leukemia therapy.
Conflict of interest statement
Figures





References
-
- Chitteti BR, Cheng YH, Poteat B, Rodriguez-Rodriguez S, Goebel WS, et al. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. 2010;115:3239–3248. blood-2009-09-246173 [pii];10.1182/blood-2009-09-246173 [doi] - PMC - PubMed
-
- Li Z, Li L. Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci. 2006;31:589–595. S0968-0004(06)00207-6 [pii];10.1016/j.tibs.2006.08.001 [doi] - PubMed
-
- Perry JM, Li L. Disrupting the stem cell niche: good seeds in bad soil. Cell. 2007;129:1045–1047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

