EGF receptor exposed to oxidative stress acquires abnormal phosphorylation and aberrant activated conformation that impairs canonical dimerization
- PMID: 21853092
- PMCID: PMC3154401
- DOI: 10.1371/journal.pone.0023240
EGF receptor exposed to oxidative stress acquires abnormal phosphorylation and aberrant activated conformation that impairs canonical dimerization
Abstract
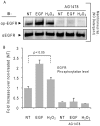
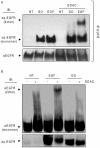
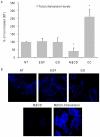
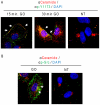
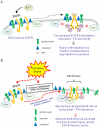
Crystallographic studies have offered understanding of how receptor tyrosine kinases from the ErbB family are regulated by their growth factor ligands. A conformational change of the EGFR (ErbB1) was shown to occur upon ligand binding, where a solely ligand-mediated mode of dimerization/activation was documented. However, this dogma of dimerization/activation was revolutionized by the discovery of constitutively active ligand-independent EGFR mutants. In addition, other ligand-independent activation mechanisms may occur. We have shown that oxidative stress (ox-stress), induced by hydrogen peroxide or cigarette smoke, activates EGFR differently than its ligand, EGF, thereby inducing aberrant phosphorylation and impaired trafficking and degradation of EGFR. Here we demonstrate that ox-stress activation of EGFR is ligand-independent, does not induce "classical" receptor dimerization and is not inhibited by the tyrosine kinase inhibitor AG1478. Thus, an unprecedented, apparently activated, state is found for EGFR under ox-stress. Furthermore, this activation mechanism is temperature-dependent, suggesting the simultaneous involvement of membrane structure. We propose that ceramide increase under ox-stress disrupts cholesterol-enriched rafts leading to EGFR re-localization into the rigid, ceramide-enriched rafts. This increase in ceramide also supports EGFR aberrant trafficking to a peri-nuclear region. Therefore, the EGFR unprecedented and activated conformation could be sustained by simultaneous alterations in membrane structure under ox-stress.
Conflict of interest statement
Figures







References
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
-
- Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. - PubMed
-
- Schlessinger J. The epidermal growth factor receptor as a multifunctional allosteric protein. Biochemistry. 1988;27:3119–3123. - PubMed
-
- Cochet C, Kashles O, Chambaz EM, Borrello I, King CR, et al. Demonstration of epidermal growth factor-induced receptor dimerization in living cells using a chemical covalent cross-linking agent. J Biol Chem. 1988;263:3290–3295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

