Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species
- PMID: 21853114
- PMCID: PMC3154436
- DOI: 10.1371/journal.pone.0023354
Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species
Abstract
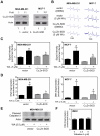
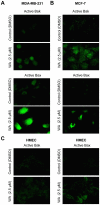
Withaferin A (WA), a promising anticancer constituent of Ayurvedic medicinal plant Withania somnifera, inhibits growth of MDA-MB-231 and MCF-7 human breast cancer cells in culture and MDA-MB-231 xenografts in vivo in association with apoptosis induction, but the mechanism of cell death is not fully understood. We now demonstrate, for the first time, that WA-induced apoptosis is mediated by reactive oxygen species (ROS) production due to inhibition of mitochondrial respiration. WA treatment caused ROS production in MDA-MB-231 and MCF-7 cells, but not in a normal human mammary epithelial cell line (HMEC). The HMEC was also resistant to WA-induced apoptosis. WA-mediated ROS production as well as apoptotic histone-associated DNA fragment release into the cytosol was significantly attenuated by ectopic expression of Cu,Zn-superoxide dismutase in both MDA-MB-231 and MCF-7 cells. ROS production resulting from WA exposure was accompanied by inhibition of oxidative phosphorylation and inhibition of complex III activity. Mitochondrial DNA-deficient Rho-0 variants of MDA-MB-231 and MCF-7 cells were resistant to WA-induced ROS production, collapse of mitochondrial membrane potential, and apoptosis compared with respective wild-type cells. WA treatment resulted in activation of Bax and Bak in MDA-MB-231 and MCF-7 cells, and SV40 immortalized embryonic fibroblasts derived from Bax and Bak double knockout mouse were significantly more resistant to WA-induced apoptosis compared with fibroblasts derived from wild-type mouse. In conclusion, the present study provides novel insight into the molecular circuitry of WA-induced apoptosis involving ROS production and activation of Bax/Bak.
Conflict of interest statement
Figures
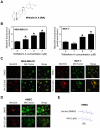

 ). (C) Effect of WA treatment on total reserve respiration capacity in MDA-MB-231 and MCF-7 cells. Total reserve respiration capacity was calculated using the mean of the time points after injection C (#11–#13) minus the mean of time points after injection D (#14–#16) (
). (C) Effect of WA treatment on total reserve respiration capacity in MDA-MB-231 and MCF-7 cells. Total reserve respiration capacity was calculated using the mean of the time points after injection C (#11–#13) minus the mean of time points after injection D (#14–#16) ( ). Data shown are mean ± SEM of three independent experiments, each performed in triplicate. *P<0.05; **P<0.01; and ***P<0.001, significantly different compared with control by one-way ANOVA with Dunnett's adjustment.
). Data shown are mean ± SEM of three independent experiments, each performed in triplicate. *P<0.05; **P<0.01; and ***P<0.001, significantly different compared with control by one-way ANOVA with Dunnett's adjustment.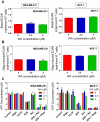



Similar articles
-
Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak.Mol Cancer Ther. 2006 Nov;5(11):2931-45. doi: 10.1158/1535-7163.MCT-06-0396. Mol Cancer Ther. 2006. PMID: 17121941
-
Autophagy fails to alter withaferin A-mediated lethality in human breast cancer cells.Curr Cancer Drug Targets. 2013 Jul;13(6):640-50. doi: 10.2174/15680096113139990039. Curr Cancer Drug Targets. 2013. PMID: 23607597 Free PMC article.
-
Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic.Breast Cancer Res Treat. 2013 Feb;138(1):69-79. doi: 10.1007/s10549-013-2440-2. Epub 2013 Feb 15. Breast Cancer Res Treat. 2013. PMID: 23412769 Free PMC article.
-
Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo.Cancer Res. 2008 Sep 15;68(18):7661-9. doi: 10.1158/0008-5472.CAN-08-1510. Cancer Res. 2008. PMID: 18794155 Free PMC article.
-
A Comprehensive Review and Perspective on Anticancer Mechanisms of Withaferin A in Breast Cancer.Cancer Prev Res (Phila). 2020 Sep;13(9):721-734. doi: 10.1158/1940-6207.CAPR-20-0259. Epub 2020 Jul 29. Cancer Prev Res (Phila). 2020. PMID: 32727824 Free PMC article. Review.
Cited by
-
NADPH-cytochrome P450 reductase: molecular cloning and functional characterization of two paralogs from Withania somnifera (L.) dunal.PLoS One. 2013;8(2):e57068. doi: 10.1371/journal.pone.0057068. Epub 2013 Feb 21. PLoS One. 2013. PMID: 23437311 Free PMC article.
-
Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development.Molecules. 2022 Jan 5;27(1):313. doi: 10.3390/molecules27010313. Molecules. 2022. PMID: 35011546 Free PMC article. Review.
-
Withaferin A inhibits adipogenesis in 3T3-F442A cell line, improves insulin sensitivity and promotes weight loss in high fat diet-induced obese mice.PLoS One. 2019 Jun 21;14(6):e0218792. doi: 10.1371/journal.pone.0218792. eCollection 2019. PLoS One. 2019. PMID: 31226166 Free PMC article.
-
Withaferin A Inhibits STAT3 and Induces Tumor Cell Death in Neuroblastoma and Multiple Myeloma.Biochem Insights. 2014 Nov 9;7:1-13. doi: 10.4137/BCI.S18863. eCollection 2014. Biochem Insights. 2014. PMID: 25452693 Free PMC article.
-
Cloning and functional characterization of three branch point oxidosqualene cyclases from Withania somnifera (L.) dunal.J Biol Chem. 2014 Jun 13;289(24):17249-67. doi: 10.1074/jbc.M114.571919. Epub 2014 Apr 25. J Biol Chem. 2014. PMID: 24770414 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. - PubMed
-
- Hulka BS, Stark AT. Breast Cancer: cause and prevention. Lancet. 1995;346:883–887. - PubMed
-
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. - PubMed
-
- Land SR, Wickerham DL, Costantino JP, Ritter MW, Vogel VG, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–2751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

