Hepatic n-3 polyunsaturated fatty acid depletion promotes steatosis and insulin resistance in mice: genomic analysis of cellular targets
- PMID: 21853118
- PMCID: PMC3154437
- DOI: 10.1371/journal.pone.0023365
Hepatic n-3 polyunsaturated fatty acid depletion promotes steatosis and insulin resistance in mice: genomic analysis of cellular targets
Abstract
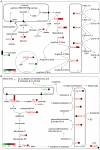
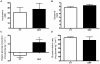
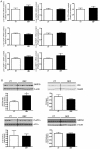
Patients with non-alcoholic fatty liver disease are characterised by a decreased n-3/n-6 polyunsaturated fatty acid (PUFA) ratio in hepatic phospholipids. The metabolic consequences of n-3 PUFA depletion in the liver are poorly understood. We have reproduced a drastic drop in n-3 PUFA among hepatic phospholipids by feeding C57Bl/6J mice for 3 months with an n-3 PUFA depleted diet (DEF) versus a control diet (CT), which only differed in the PUFA content. DEF mice exhibited hepatic insulin resistance (assessed by euglycemic-hyperinsulinemic clamp) and steatosis that was associated with a decrease in fatty acid oxidation and occurred despite a higher capacity for triglyceride secretion. Microarray and qPCR analysis of the liver tissue revealed higher expression of all the enzymes involved in lipogenesis in DEF mice compared to CT mice, as well as increased expression and activation of sterol regulatory element binding protein-1c (SREBP-1c). Our data suggest that the activation of the liver X receptor pathway is involved in the overexpression of SREBP-1c, and this phenomenon cannot be attributed to insulin or to endoplasmic reticulum stress responses. In conclusion, n-3 PUFA depletion in liver phospholipids leads to activation of SREBP-1c and lipogenesis, which contributes to hepatic steatosis.
Conflict of interest statement
Figures

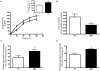

Similar articles
-
Endoplasmic reticulum stress leads to lipid accumulation through upregulation of SREBP-1c in normal hepatic and hepatoma cells.Mol Cell Biochem. 2013 Sep;381(1-2):127-37. doi: 10.1007/s11010-013-1694-7. Epub 2013 May 24. Mol Cell Biochem. 2013. PMID: 23703028
-
Prebiotic approach alleviates hepatic steatosis: implication of fatty acid oxidative and cholesterol synthesis pathways.Mol Nutr Food Res. 2013 Feb;57(2):347-59. doi: 10.1002/mnfr.201200364. Epub 2012 Dec 2. Mol Nutr Food Res. 2013. PMID: 23203768
-
Hepatic steatosis in n-3 fatty acid depleted mice: focus on metabolic alterations related to tissue fatty acid composition.BMC Physiol. 2008 Dec 1;8:21. doi: 10.1186/1472-6793-8-21. BMC Physiol. 2008. PMID: 19046413 Free PMC article.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
-
Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c.Diabetes Obes Metab. 2010 Oct;12 Suppl 2:83-92. doi: 10.1111/j.1463-1326.2010.01275.x. Diabetes Obes Metab. 2010. PMID: 21029304 Review.
Cited by
-
Lipidomic profiling of the hepatic esterified fatty acid composition in diet-induced nonalcoholic fatty liver disease in genetically diverse Collaborative Cross mice.J Nutr Biochem. 2022 Nov;109:109108. doi: 10.1016/j.jnutbio.2022.109108. Epub 2022 Jul 17. J Nutr Biochem. 2022. PMID: 35858665 Free PMC article.
-
Comprehensive lifestyle intervention vs soy protein-based meal regimen in non-alcoholic steatohepatitis.World J Gastroenterol. 2019 Mar 7;25(9):1116-1131. doi: 10.3748/wjg.v25.i9.1116. World J Gastroenterol. 2019. PMID: 30862999 Free PMC article. Clinical Trial.
-
The Effects of Maternal Intake of EPA and DHA Enriched Diet During Pregnancy and Lactation on Offspring's Muscle Development and Energy Homeostasis.Front Physiol. 2022 Jun 6;13:881624. doi: 10.3389/fphys.2022.881624. eCollection 2022. Front Physiol. 2022. PMID: 35733999 Free PMC article.
-
Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice.Lipids. 2014 May;49(5):431-44. doi: 10.1007/s11745-014-3892-9. Epub 2014 Mar 14. Lipids. 2014. PMID: 24627299
-
Omega-3 fatty acids for the treatment of non-alcoholic fatty liver disease.World J Gastroenterol. 2012 Nov 7;18(41):5839-47. doi: 10.3748/wjg.v18.i41.5839. World J Gastroenterol. 2012. PMID: 23139599 Free PMC article. Review.
References
-
- Clarke SD. Nonalcoholic steatosis and steatohepatitis. I. Molecular mechanism for polyunsaturated fatty acid regulation of gene transcription. Am J Physiol Gastrointest Liver Physiol. 2001;281:G865–G869. - PubMed
-
- Pawar A, Jump DB. Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor alpha activity in rat primary hepatocytes. J Biol Chem. 2003;278:35931–35939. - PubMed
-
- Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. - PubMed
-
- Sekiya M, Yahagi N, Matsuzaka T, Najima Y, Nakakuki M, et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–1539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

