Increased endothelial cell-leukocyte interaction in murine schistosomiasis: possible priming of endothelial cells by the disease
- PMID: 21853150
- PMCID: PMC3154496
- DOI: 10.1371/journal.pone.0023547
Increased endothelial cell-leukocyte interaction in murine schistosomiasis: possible priming of endothelial cells by the disease
Abstract
Background and aims: Schistosomiasis is an intravascular parasitic disease associated with inflammation. Endothelial cells control leukocyte transmigration and vascular permeability being modulated by pro-inflammatory mediators. Recent data have shown that endothelial cells primed in vivo in the course of a disease keep the information in culture. Herein, we evaluated the impact of schistosomiasis on endothelial cell-regulated events in vivo and in vitro.
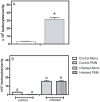
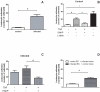
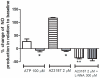
Methodology and principal findings: The experimental groups consisted of Schistosoma mansoni-infected and age-matched control mice. In vivo infection caused a marked influx of leukocytes and an increased protein leakage in the peritoneal cavity, characterizing an inflamed vascular and cellular profile. In vitro leukocyte-mesenteric endothelial cell adhesion was higher in cultured cells from infected mice as compared to controls, either in the basal condition or after treatment with the pro-inflammatory cytokine tumor necrosis factor (TNF). Nitric oxide (NO) donation reduced leukocyte adhesion to endothelial cells from control and infected groups; however, in the later group the effect was more pronounced, probably due to a reduced NO production. Inhibition of control endothelial NO synthase (eNOS) increased leukocyte adhesion to a level similar to the one observed in the infected group. Besides, the adhesion of control leukocytes to endothelial cells from infected animals is similar to the result of infected animals, confirming that schistosomiasis alters endothelial cells function. Furthermore, NO production as well as the expression of eNOS were reduced in cultured endothelial cells from infected animals. On the other hand, the expression of its repressor protein, namely caveolin-1, was similar in both control and infected groups.
Conclusion/significance: Schistosomiasis increases vascular permeability and endothelial cell-leukocyte interaction in vivo and in vitro. These effects are partially explained by a reduced eNOS expression. In addition, our data show that the disease primes endothelial cells in vivo, which keep the acquired phenotype in culture.
Conflict of interest statement
Figures
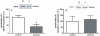

Similar articles
-
Increased expression of NTPDases 2 and 3 in mesenteric endothelial cells during schistosomiasis favors leukocyte adhesion through P2Y1 receptors.Vascul Pharmacol. 2016 Jul;82:66-72. doi: 10.1016/j.vph.2016.02.005. Epub 2016 Feb 24. Vascul Pharmacol. 2016. PMID: 26924460
-
Endothelial P2X7 receptors' expression is reduced by schistosomiasis.Purinergic Signal. 2013 Mar;9(1):81-9. doi: 10.1007/s11302-012-9332-5. Epub 2012 Sep 18. Purinergic Signal. 2013. PMID: 22987361 Free PMC article.
-
Endothelial [Ca2+]i and caveolin-1 antagonistically regulate eNOS activity and microvessel permeability in rat venules.Cardiovasc Res. 2010 Jul 15;87(2):340-7. doi: 10.1093/cvr/cvq006. Epub 2010 Jan 15. Cardiovasc Res. 2010. PMID: 20080986 Free PMC article.
-
Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells.J Hypertens. 2010 May;28(5):940-51. doi: 10.1097/HJH.0b013e32833992ef. J Hypertens. 2010. PMID: 20375905
-
Interaction and involvement of cellular adhesion molecules in the pathogenesis of Schistosomiasis mansoni.Immunol Lett. 2019 Feb;206:11-18. doi: 10.1016/j.imlet.2018.11.011. Epub 2018 Nov 29. Immunol Lett. 2019. PMID: 30503821 Review.
Cited by
-
Interference with the host haemostatic system by schistosomes.PLoS Pathog. 2013;9(12):e1003781. doi: 10.1371/journal.ppat.1003781. Epub 2013 Dec 26. PLoS Pathog. 2013. PMID: 24385897 Free PMC article. Review.
-
P2Y2-P2X7 receptors cross-talk in primed mesenteric endothelial cells upregulates NF-κB signaling favoring mononuclear cell adhesion in schistosomiasis.Front Immunol. 2024 Jan 4;14:1328897. doi: 10.3389/fimmu.2023.1328897. eCollection 2023. Front Immunol. 2024. PMID: 38239348 Free PMC article.
-
Purinergic signaling in schistosomal infection.Biomed J. 2016 Oct;39(5):316-325. doi: 10.1016/j.bj.2016.06.006. Epub 2016 Nov 3. Biomed J. 2016. PMID: 27884378 Free PMC article. Review.
-
Transcriptomic Remodelling of Fetal Endothelial Cells During Establishment of Inflammatory Memory.Front Immunol. 2021 Nov 19;12:757393. doi: 10.3389/fimmu.2021.757393. eCollection 2021. Front Immunol. 2021. PMID: 34867995 Free PMC article.
-
Injury-Induced Shedding of Extracellular Vesicles Depletes Endothelial Cells of Cav-1 (Caveolin-1) and Enables TGF-β (Transforming Growth Factor-β)-Dependent Pulmonary Arterial Hypertension.Arterioscler Thromb Vasc Biol. 2019 Jun;39(6):1191-1202. doi: 10.1161/ATVBAHA.118.312038. Arterioscler Thromb Vasc Biol. 2019. PMID: 30943774 Free PMC article.
References
-
- Silva CLM, Morel N, Lenzi HL, Noël F. Increased reactivity to 5-hydroxytryptamine of portal veins from mice infected with Schistosoma mansoni. . Comp Biochem Physiol (A) 1998;120:417–423. - PubMed
-
- Angeli V, Faveeuw C, Delerive P, Fontaine J, Barriera Y, et al. Schistosoma mansoni induces the synthesis of IL-6 in pulmonary microvascular endothelial cells: role of IL-6 in the control of lung eosinophilia during infection. Eur J Immunol. 2001;31:2751–2761. - PubMed
-
- Loeffler DA, Lundy SK, Singh KP, Gerard HC, Hudson AP, et al. Soluble egg antigens from Schistosoma mansoni induce angiogenesis-related processes by up-regulating vascular endothelial growth factor in human endothelial cells. J Inf Dis. 2002;185:1650–1656. - PubMed
-
- Andrade ZA. Schistosomiasis and liver fibrosis. Parasite Immunol. 2009;31:656–663. - PubMed
-
- Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

