In vitro and in vivo activity of ribavirin against Andes virus infection
- PMID: 21853152
- PMCID: PMC3154477
- DOI: 10.1371/journal.pone.0023560
In vitro and in vivo activity of ribavirin against Andes virus infection
Abstract
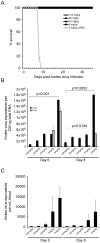
Pathogenic hantaviruses are a closely related group of rodent-borne viruses which are responsible for two distinct diseases in humans, hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome (HPS, otherwise known as hantavirus cardiopulmonary syndrome, HCPS). The antiviral effect of ribavirin against Old World hantaviruses, most notably Hantaan virus, is well documented; however, only a few studies have addressed its inhibitory effect on New World hantaviruses. In the present study, we demonstrate that ribavirin is highly active against Andes virus (ANDV), an important etiological agent of HPS, both in vitro and in vivo using a lethal hamster model of HPS. Treatment of ANDV infected Vero E6 cells with ribavirin resulted in dose-dependent reductions in viral RNA and protein as well as virus yields with a half maximal inhibitory concentration between 5 and 12.5 µg ml(-1). In hamsters, treatment with as little as 5 mg kg(-1) day(-1) was 100% effective at preventing lethal HPS disease when therapy was administered by intraperitoneal injection from day 1 through day 10 post-infection. Significant reductions were observed in ANDV RNA and antigen positive cells in lung and liver tissues. Ribavirin remained completely protective when administered by intraperitoneal injections up to three days post-infection. In addition, we show that daily oral ribavirin therapy initiated 1 day post-infection and continuing for ten days is also protective against lethal ANDV disease, even at doses of 5 mg kg(-1) day(-1). Our results suggest ribavirin treatment is beneficial for postexposure prophylaxis against HPS-causing hantaviruses and should be considered in scenarios where exposure to the virus is probable. The similarities between the results obtained in this study and those from previous clinical evaluations of ribavirin against HPS, further validate the hamster model of lethal HPS and demonstrate its usefulness in screening antiviral agents against this disease.
Conflict of interest statement
Figures



References
-
- Nichol ST, Elliott RM, Goldbach R, Plyusnin A, Schmaljohn CS, et al. Bunyaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy, VIIIth Report of the International Committee on Taxonomy of Viruses. London: Elsevier Academic Press; 2005. pp. 695–716.
-
- Padula PJ, Edelstein A, Miquel SD, Lopez NM, Rossi CM, et al. Hantavirus pulmonary syndrome outbreak in Argentina: molecular evidence for person-to-person transmission of Andes virus. Virol. 1998;241:323–330. - PubMed
-
- Schmaljohn C. Vaccines for hantaviruses. Vaccine. 2009;27:D61–D64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

