Cell receptor and surface ligand density effects on dynamic states of adhering circulating tumor cells
- PMID: 21853194
- PMCID: PMC6765388
- DOI: 10.1039/c1lc20455f
Cell receptor and surface ligand density effects on dynamic states of adhering circulating tumor cells
Abstract
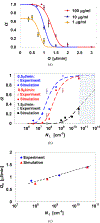
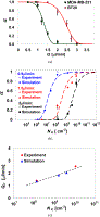
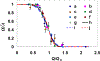
Dynamic states of cancer cells moving under shear flow in an antibody-functionalized microchannel are investigated experimentally and theoretically. The cell motion is analyzed with the aid of a simplified physical model featuring a receptor-coated rigid sphere moving above a solid surface with immobilized ligands. The motion of the sphere is described by the Langevin equation accounting for the hydrodynamic loadings, gravitational force, receptor-ligand bindings, and thermal fluctuations; the receptor-ligand bonds are modeled as linear springs. Depending on the applied shear flow rate, three dynamic states of cell motion have been identified: (i) free motion, (ii) rolling adhesion, and (iii) firm adhesion. Of particular interest is the fraction of captured circulating tumor cells, defined as the capture ratio, via specific receptor-ligand bonds. The cell capture ratio decreases with increasing shear flow rate with a characteristic rate. Based on both experimental and theoretical results, the characteristic flow rate increases monotonically with increasing either cell-receptor or surface-ligand density within certain ranges. Utilizing it as a scaling parameter, flow-rate dependent capture ratios for various cell-surface combinations collapse onto a single curve described by an exponential formula.
Figures




References
-
- Nguyen DX and Massagué J, Genetic determinants of cancer metastasis. Nat. Rev. Genet, vol. 8, pp. 341–352, 2007. - PubMed
-
- Gupta GP and Massagué J, Cancer metastasis: building a framework. Cell, vol. 127, pp. 679–695, 2006. - PubMed
-
- Lawrence MB and Springer TA, Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell, vol. 65, pp. 859–873, 1991. - PubMed
-
- Dong C, Cao J, Struble EJ and Lipowsky HH, Mechanics of leukocyte deformation and adhesion to endothelium in shear flow. Ann. Biomed. Eng, vol. 27, pp. 298–312, 1999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

