Identification of proline residues in or near the transmembrane helices of the human breast cancer resistance protein (BCRP/ABCG2) that are important for transport activity and substrate specificity
- PMID: 21854076
- PMCID: PMC3172388
- DOI: 10.1021/bi200573t
Identification of proline residues in or near the transmembrane helices of the human breast cancer resistance protein (BCRP/ABCG2) that are important for transport activity and substrate specificity
Abstract
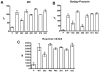
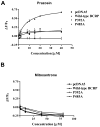
The human breast cancer resistance protein (BCRP/ABCG2) confers multidrug resistance and mediates the active efflux of drugs and xenobiotics. BCRP contains one nucleotide-binding domain (NBD) followed by one membrane-spanning domain (MSD). We investigated whether prolines in or near the transmembrane helices are essential for BCRP function. Six proline residues were substituted with alanine individually, and the mutants were stably expressed in Flp-In(TM)-293 cells at levels comparable to that of wild-type BCRP and predominantly localized on the plasma membrane of the cells. While P392A showed a significant reduction (35-50%) in the efflux activity of mitoxantrone, BODIPY-prazosin, and Hoechst 33342, P485A exhibited a significant decrease of approximately 70% in the efflux activity of only BODIPY-prazosin. Other mutants had no significant changes in the efflux activities of these substrates. Drug resistance profiles of the cells expressing the mutants correlated well with the efflux data. ATPase activity was not substantially affected for P392A or P485A compared to that of wild-type BCRP. These results strongly suggest Pro(392) and Pro(485) are important in determining the overall transport activity and substrate selectivity of BCRP, respectively. Prazosin differentially affected the binding of 5D3, a conformation-sensitive antibody, to wild-type BCRP, P392A, or P485A in a concentration-dependent manner. In contrast, mitoxantrone had no significant effect on 5D3 binding. Homology modeling indicates that Pro(392) may play an important role in the communication between the MSD and NBD as it is predicted to be located at the interface between the two functional domains, and Pro(485) induces flexible hinges that may be essential for the broad substrate specificity of BCRP.
© 2011 American Chemical Society
Figures





References
-
- Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–1236. - PubMed
-
- Ishikawa T, Nakagawa H. Human ABC transporter ABCG2 in cancer chemotherapy and pharmacogenomics. J Exp Ther Oncol. 2009;8:5–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

