Design of NIDA CTN Protocol 0047: screening, motivational assessment, referral, and treatment in emergency departments (SMART-ED)
- PMID: 21854285
- PMCID: PMC3168577
- DOI: 10.3109/00952990.2011.596971
Design of NIDA CTN Protocol 0047: screening, motivational assessment, referral, and treatment in emergency departments (SMART-ED)
Abstract
Background: Medical settings such as emergency departments (EDs) present an opportunity to identify and provide services for individuals with substance use problems who might otherwise never receive any form of assessment, referral, or intervention. Although screening, brief intervention, and referral to treatment models have been extensively studied and are considered effective for individuals with alcohol problems presenting in EDs and other medical settings, the efficacy of such interventions has not been established for drug users presenting in EDs.
Objectives: This article describes the design of a NIDA Clinical Trials Network protocol testing the efficacy of an screening, brief intervention, and referral to treatment model in medical EDs, highlighting considerations that are pertinent to the design of other studies targeting substance use behaviors in medical treatment settings.
Methods: The protocol is described, and critical design decisions are discussed.
Results: Design challenges included defining treatment conditions, study population, and site characteristics; developing the screening process; choosing the primary outcome; balancing brevity and comprehensiveness of assessment; and selecting the strategy for statistical analysis.
Conclusion: Many of the issues arising in the design of this study will be relevant to future studies of interventions for addictions in medical settings.
Scientific significance: Optimal trial design is critical to determining how best to integrate substance abuse interventions into medical care.
Figures

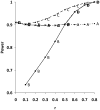
A - Analyze 3-month outcomes only.
B - Analyze change only.
C - Use baseline as a fixed-effect covariate to analyze 3-month outcomes.
D - At trial’s end, calculate the average pre-post correlation within the two treatment groups. If this exceeds 0.5, analyze changes. If it is less than 0.5, analyze 3-mo outcome.
References
-
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA: Journal of the American Medical Association. 2004;291(10):1238–45. - PubMed
-
- Rockett IRH, Putnam SL, Jia H, Chang CF, Smith GS. Unmet substance abuse treatment need, health services utilization, and cost: A population-based emergency department study. Annals of emergency medicine. 2005;45(2):118–27. - PubMed
-
- Rockett IRH, Putnam SL, Jia H, Smith GS. Declared and undeclared substance use among emergency department patients: a population-based study. Addiction. 2006;101(5):706–12. - PubMed
-
- Calle PA, Damen J, De Paepe P, Monsieurs KG, Buylaert WA, Belg AC. A survey on alcohol and illicit drug abuse among emergency department patients. Acta Clinica Belgica. 2006;61(4):188–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
