Nanoparticles for transcutaneous vaccination
- PMID: 21854553
- PMCID: PMC3815776
- DOI: 10.1111/j.1751-7915.2011.00284.x
Nanoparticles for transcutaneous vaccination
Abstract
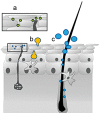
The living epidermis and dermis are rich in antigen presenting cells (APCs). Their activation can elicit a strong humoral and cellular immune response as well as mucosal immunity. Therefore, the skin is a very attractive site for vaccination, and an intradermal application of antigen may be much more effective than a subcutaneous or intramuscular injection. However, the stratum corneum (SC) is a most effective barrier against the invasion of topically applied vaccines. Products which have reached the stage of clinical testing, avoid this problem by injecting the nano-vaccine intradermally or by employing a barrier disrupting method and applying the vaccine to a relatively large skin area. Needle-free vaccination is desirable from a number of aspects: ease of application, improved patient acceptance and less risk of infection among them. Nanocarriers can be designed in a way that they can overcome the SC. Also incorporation into nanocarriers protects instable antigen from degradation, improves uptake and processing by APCs, and facilitates endosomal escape and nuclear delivery of DNA vaccines. In addition, sustained release systems may build a depot in the tissue gradually releasing antigen which may avoid booster doses. Therefore, nanoformulations of vaccines for transcutaneous immunization are currently a very dynamic field of research. Among the huge variety of nanocarrier systems that are investigated hopes lie on ultra-flexible liposomes, superfine rigid nanoparticles and nanocarriers, which are taken up by hair follicles. The potential and pitfalls associated with these three classes of carriers will be discussed.
© 2011 The Authors; Microbial Biotechnology © 2011 Society for Applied Microbiology and Blackwell Publishing Ltd.
Figures
References
-
- Antonelli A., Ferri C., Ferrari S.M., Colaci M., Sansonno D., Fallahi P. Endocrine manifestations of hepatitis C virus infection. Nat Clin Pract Endocrinol Metab. 2009;5:26–34. - PubMed
-
- Apple R.J., Erlich H.A., Klitz W., Manos M.M., Becker T.M., Wheeler C.M. HLA DR‐DQ associations with cervical carcinoma show papillomavirus‐type specificity. Nat Gen. 1994;6:157–162. - PubMed
-
- Babiuk S., Baca‐Estrada M., Babiuk L.A., Ewen C., Foldvari M. Cutaneous vaccination: the skin as an immunologically active tissue and the challenge of antigen delivery. J Control Release. 2000;66:199–214. - PubMed
-
- Bal S.M., Slutter B., van Riet E., Kruithof A.C., Ding Z., Kersten G.F. Efficient induction of immune responses through intradermal vaccination with N‐trimethyl chitosan containing antigen formulations. J Control Release. 2010;142:374–383. et al. - PubMed
-
- Bond J.R., Barry B.W. Hairless mouse skin is limited as a model for assessing the effects of penetration enhancers in human skin. J Invest Dermatol. 1988;90:810–813. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

