The BH3 mimetic ABT-737 increases treatment efficiency of paclitaxel against hepatoblastoma
- PMID: 21854558
- PMCID: PMC3176244
- DOI: 10.1186/1471-2407-11-362
The BH3 mimetic ABT-737 increases treatment efficiency of paclitaxel against hepatoblastoma
Abstract
Background: The primary goal of current chemotherapy in hepatoblastoma (HB) is reduction of tumour volume and vitality to enable complete surgical resection and reduce risk of recurrence or metastatic disease. Drug resistance remains a major challenge for HB treatment. In some malignancies inhibition of anti-apoptotic pathways using small BH3 mimetic molecules like ABT-737 shows synergistic effects in combination with cystotoxic agents in vitro. Now we analysed toxicology and synergistic effects of this approach in HB cells and HB xenografts.
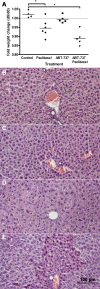
Methods: Viability was monitored in HB cells (HUH6 and HepT1) and fibroblasts treated with paclitaxel, ABT-737 and a combination of both in a MTT assay. HUH6 xenotransplants in NOD/LtSz-scid IL2Rγnull mice (NSG) were treated accordingly. Tumour volume and body weight were monitored. Xenografted tumours were analysed by histology and immunohistochemistry (Ki-67 and TUNEL assay).
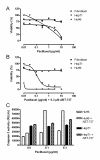
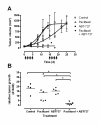
Results: ABT-737 reduced viability in HUH6 and HepT1 cells cultures at concentrations above 1 μM and also enhanced the cytotoxic effect of paclitaxel when used in combination. Thereby paclitaxel could be reduced tenfold to achieve similar reduction of viability of tumour cells. In contrast no toxicity in fibroblasts was observed at the same regiments. Subcutaneous HB (HUH6) treated with paclitaxel (12 mg/kg body weight, n = 7) led to delayed tumour growth in the beginning of the experiment. However, tumour volume was similar to controls (n = 5) at day 25. Combination treatment with paclitaxel and ABT-737 (100 mg/kg, n = 8) revealed significantly 10 fold lower relative tumour volumes compared to control and paclitaxel groups. Paclitaxel dependent toxicity was observed in this mice strain.
Conclusions: Our results demonstrate enhancement of chemotherapy by using modulators of apoptosis. Further analyses should include improved pharmacological formulations of paclitaxel and BH3 mimetics in order to reduce toxicological effects. Sensitising HB to apoptosis may also render resistant HB susceptible to established chemotherapy regimens.
Figures

Similar articles
-
Increased efficacy of CDDP in a xenograft model of hepatoblastoma using the apoptosis sensitizer ABT-737.Oncol Rep. 2013 Feb;29(2):646-52. doi: 10.3892/or.2012.2150. Epub 2012 Nov 27. Oncol Rep. 2013. PMID: 23229825
-
BH3-mimetic drugs prevent tumour onset in an orthotopic mouse model of hepatoblastoma.Exp Cell Res. 2014 Mar 10;322(1):217-25. doi: 10.1016/j.yexcr.2013.12.007. Epub 2013 Dec 16. Exp Cell Res. 2014. PMID: 24355809
-
Inhibition of Bcl-2 and Bcl-X enhances chemotherapy sensitivity in hepatoblastoma cells.Pediatr Blood Cancer. 2010 Dec 1;55(6):1089-95. doi: 10.1002/pbc.22740. Pediatr Blood Cancer. 2010. PMID: 20680965
-
The role of BH3-mimetic drugs in the treatment of pediatric hepatoblastoma.Int J Mol Sci. 2015 Feb 16;16(2):4190-208. doi: 10.3390/ijms16024190. Int J Mol Sci. 2015. PMID: 25690034 Free PMC article. Review.
-
Small molecule inhibition of the Bcl-X(L)-BH3 protein-protein interaction: proof-of-concept of an in vivo chemopotentiator ABT-737.Curr Top Med Chem. 2007;7(10):961-5. doi: 10.2174/156802607780906843. Curr Top Med Chem. 2007. PMID: 17508927 Review.
Cited by
-
Regulating the BCL2 Family to Improve Sensitivity to Microtubule Targeting Agents.Cells. 2019 Apr 12;8(4):346. doi: 10.3390/cells8040346. Cells. 2019. PMID: 31013740 Free PMC article. Review.
-
Effect of duplex drugs linking 2'-deoxy-5-fluorouridine (5-FdU) with 3'-C-ethynylcytidine (ECyd) on hepatoblastoma cell lines.Pediatr Surg Int. 2013 Feb;29(2):121-7. doi: 10.1007/s00383-012-3192-5. Pediatr Surg Int. 2013. PMID: 23187893
-
Hepatoblastoma: A Need for Cell Lines and Tissue Banks to Develop Targeted Drug Therapies.Front Pediatr. 2016 Mar 21;4:22. doi: 10.3389/fped.2016.00022. eCollection 2016. Front Pediatr. 2016. PMID: 27047905 Free PMC article. Review.
-
A germline-competent embryonic stem cell line from NOD.Cg-Prkdc ( scid ) Il2rg ( tm1Wjl )/SzJ (NSG) mice.Transgenic Res. 2013 Feb;22(1):179-85. doi: 10.1007/s11248-012-9629-8. Epub 2012 Jul 6. Transgenic Res. 2013. PMID: 22767020
-
AMPK and PFKFB3 mediate glycolysis and survival in response to mitophagy during mitotic arrest.Nat Cell Biol. 2015 Oct;17(10):1304-16. doi: 10.1038/ncb3231. Epub 2015 Aug 31. Nat Cell Biol. 2015. PMID: 26322680
References
-
- Fuchs J, Rydzynski J, Von Schweinitz D, Bode U, Hecker H, Weinel P, Burger D, Harms D, Erttmann R, Oldhafer K, Mildenberger H. Pretreatment prognostic factors and treatment results in children with hepatoblastoma: a report from the German Cooperative Pediatric Liver Tumor Study HB 94. Cancer. 2002;95(1):172–182. doi: 10.1002/cncr.10632. - DOI - PubMed
-
- Perilongo G, Shafford E, Maibach R, Aronson D, Brugieres L, Brock P, Childs M, Czauderna P, MacKinlay G, Otte JB, Pritchard J, Rondelli R, Scopinaro M, Staalman C, Plaschkes J. Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology--SIOPEL 2. Eur J Cancer. 2004;40(3):411–421. doi: 10.1016/j.ejca.2003.06.003. - DOI - PubMed
-
- Zsiros J, Maibach R, Shafford E, Brugieres L, Brock P, Czauderna P, Roebuck D, Childs M, Zimmermann A, Laithier V, Otte JB, de Camargo B, MacKinlay G, Scopinaro M, Aronson D, Plaschkes J, Perilongo G. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol. 2009;28(15):2584–2590. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

