Reduction of neutrophil activity decreases early microvascular injury after subarachnoid haemorrhage
- PMID: 21854561
- PMCID: PMC3170601
- DOI: 10.1186/1742-2094-8-103
Reduction of neutrophil activity decreases early microvascular injury after subarachnoid haemorrhage
Abstract
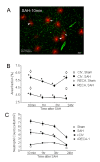
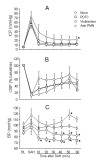
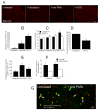
Background: Subarachnoid haemorrhage (SAH) elicits rapid pathological changes in the structure and function of parenchymal vessels (≤ 100 μm). The role of neutrophils in these changes has not been determined. This study investigates the role of neutrophils in early microvascular changes after SAH METHOD: Rats were either untreated, treated with vinblastine or anti-polymorphonuclear (PMN) serum, which depletes neutrophils, or treated with pyrrolidine dithiocarbamate (PDTC), which limits neutrophil activity. SAH was induced by endovascular perforation. Neutrophil infiltration and the integrity of vascular endothelium and basement membrane were assessed immunohistochemically. Vascular collagenase activity was assessed by in situ zymography.
Results: Vinblastine and anti-PMN serum reduced post-SAH accumulation of neutrophils in cerebral vessels and in brain parenchyma. PDTC increased the neutrophil accumulation in cerebral vessels and decreased accumulation in brain parenchyma. In addition, each of the three agents decreased vascular collagenase activity and post-SAH loss of vascular endothelial and basement membrane immunostaining.
Conclusions: Our results implicate neutrophils in early microvascular injury after SAH and indicate that treatments which reduce neutrophil activity can be beneficial in limiting microvascular injury and increasing survival after SAH.
Figures


References
-
- Scholler K, Trinkl A, Klopotowski M, Thal SC, Plesnila N, Trabold R, Hamann GF, Schmid-Elsaesser R, Zausinger S. Characterization of microvascular basal lamina damage and blood-brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007;1142:237–246. - PubMed
-
- Park KW, Metais C, Dai HB, Comunale ME, Sellke FW. Microvascular endothelial dysfunction and its mechanism in a rat model of subarachnoid hemorrhage. Anesth Analg. 2001;92:990–996. pp. 990-996.; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

