Modeling oculopharyngeal muscular dystrophy in myotube cultures reveals reduced accumulation of soluble mutant PABPN1 protein
- PMID: 21854744
- PMCID: PMC3181361
- DOI: 10.1016/j.ajpath.2011.06.044
Modeling oculopharyngeal muscular dystrophy in myotube cultures reveals reduced accumulation of soluble mutant PABPN1 protein
Abstract
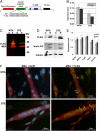
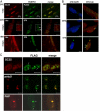
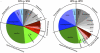
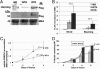
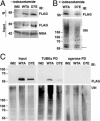
Oculopharyngeal muscular dystrophy (OPMD) is an autosomal dominant disease caused by an alanine tract expansion mutation in poly(A) binding protein nuclear 1 (expPABPN1). To model OPMD in a myogenic and physiological context, we generated mouse myoblast cell clones stably expressing either human wild type (WT) or expPABPN1 at low levels. Transgene expression is induced on myotube differentiation and results in formation of insoluble nuclear PABPN1 aggregates that are similar to those observed in patients with OPMD. Quantitative analysis of PABPN1 in myotube cultures revealed that expPABPN1 accumulation and aggregation is greater than that of the WT protein. We found that aggregation of expPABPN1 is more affected than WT PABPN1 by inhibition of proteasome activity. Consistent with this, in myotube cultures expressing expPABPN1, deregulation of the proteasome was identified as the most significantly perturbed pathway. Differences in the accumulation of soluble WT and expPABPN1 were consistent with differences in ubiquitination and rate of protein turnover. This study demonstrates, for the first time to our knowledge, that, in myotubes, the ratio of soluble/insoluble expPABPN1 is significantly lower compared with that of the WT protein. We suggest that this difference can contribute to muscle weakness in OPMD.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

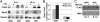
References
-
- Cummings C.J., Zoghbi H.Y. Fourteen and counting: unraveling trinucleotide repeat diseases. Hum Mol Genet. 2000;9:909–916. - PubMed
-
- Albrecht A., Mundlos S. The other trinucleotide repeat: polyalanine expansion disorders. Curr Opin Genet Dev. 2005;15:285–293. - PubMed
-
- Brown L.Y., Brown S.A. Alanine tracts: the expanding story of human illness and trinucleotide repeats. Trends Genet. 2004;20:51–58. - PubMed
-
- Ross C.A., Poirier M.A. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–S17. - PubMed
-
- Brais B., Bouchard J.P., Xie Y.G., Rochefort D.L., Chretien N., Tome F.M., Lafreniere R.G., Rommens J.M., Uyama E., Nohira O., Blumen S., Korczyn A.D., Heutink P., Mathieu J., Duranceau A., Codere F., Fardeau M., Rouleau G.A. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18:164–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

